During the coronavirus disease 2019 (COVID-19) pandemic, many hospitals implemented admission testing for severe acute respiratory coronavirus virus 2 (SARS-CoV-2) using reverse-transcription quantitative polymerase chain reaction (RT-qPCR) on all patients to evaluate the need for transmission-based precautions. As a result, asymptomatic patients with a positive test often experienced delays in medical care for the condition that brought them into the hospital. Reference Larsen, Bub, Schaffler, Walden and Intravia1 Multiple prior studies have examined the relationship between SARS-CoV-2 RT-qPCR cycle threshold (Ct) values and the ability to isolate virus in culture, which indicates viable virus with the potential for transmission. In general, the higher the Ct, the less likely that the virus would grow in culture, indicating a lack of ongoing infectiousness. Reference Bullard, Dust and Funk2,Reference Singanayagam, Patel and Charlett3 At our academic, tertiary-care hospital, a strand-specific RT-qPCR test was developed and validated that detects SARS-CoV-2 minus-strand ribonucleic acid (RNA) as a proxy for actively replicating virus with transmission potential. Reference Hogan, Huang and Sahoo4 The strand assay could be ordered by any treating physician and was commonly used for asymptomatic patients or before removal of isolation in profoundly immunocompromised patients. By evaluating minus-strand results, we identified rates of viral replication and presumed infectiousness among asymptomatic patients with a positive admission test for SARS-CoV-2.
Methods
We used an RT-qPCR specific to the minus strand of the SARS-CoV-2 envelope gene. Data were collected electronically from the Epic Clarity database (Epic, Verona, WI) for patients with a positive SARS-CoV-2 admission PCR test who were also tested using the strand-specific SARS-CoV-2 RT-qPCR within 2 days of admission at Stanford Health Care between August 2020 and April 2022. We performed a chart review to extract baseline characteristics including presence of symptoms. We restricted our analysis to each patient’s first test. We calculated the percentage of detectable minus-strand RNA results among asymptomatic patients over time, and we calculated descriptive statistics for baseline demographics, immunocompromising conditions, and reasons for testing.
Results
In total, 1,064 strand-specific SARS-CoV-2 RT-qPCR tests were performed on 848 admitted patients. Of 389 tests performed on asymptomatic patients, 242 were collected within 2 days of admission (Fig. S1). Among the asymptomatic patients, the mean age was 56 years (range, 19–99 years) and 133 (55%) were male. Furthermore, 50 patients (21%) had immunocompromising conditions: 12 patients with solid-organ transplants, 15 patients who received chimeric antigen receptor T-cell (CAR-T) therapy or bone-marrow transplant (BMT) or who were on active chemotherapy, and 23 patients with other immunocompromising conditions including those on steroid treatment, those with HIV/AIDS, and those with primary immunodeficiency. Also, 30 patients (12%) were admitted to the hospital for a surgical procedure. Among the 242 asymptomatic patients tested within 2 days of admission, only 21 patients had detectable minus-strand RNA (9%; range, 4%–25% per quarter) (Fig. 1). Among the patients with detectable minus-strand RNA, 4 (19%) were immunocompromised, and none required admission to the ICU.
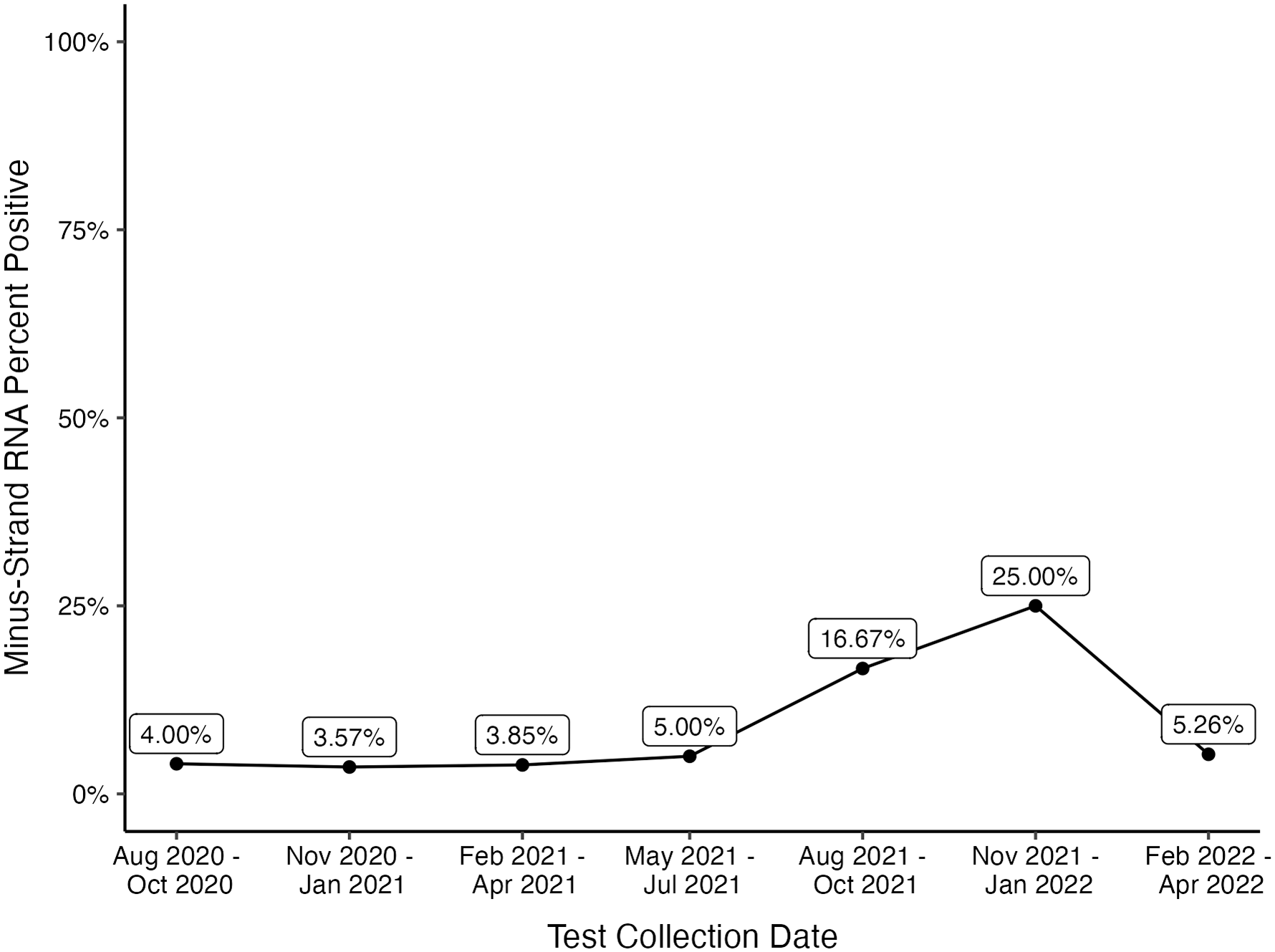
Figure 1. Percent positivity of minus-strand RNA per quarter among asymptomatic patients tested within 2 days of admission. The peak positivity in November 2021–January 2022 quarter coincided with the SARS-CoV-2 omicron variant surge.
Discussion
In this single-center retrospective study of asymptomatic patients with positive SARS-CoV-2 admission tests, most patients who had a strand-specific RT-qPCR test lacked replicating virus and therefore were presumed to be noninfectious. Our findings support the discontinuation of admission testing of asymptomatic patients. Healthcare facilities should continue emphasizing other horizontal infection controls such as ventilation, vaccination, and early detection of symptomatic cases.
In this cohort, only 21 patients (9%) had detectable minus-strand RNA (range, 4%–25% per quarter) within 48 hours of their admission testing. The highest positivity (25%) correlated with the introduction of the SARS-CoV-2 Omicron variant. These findings agree with prior studies that identified positive SARS-CoV-2 RT-qPCR tests without the presence of culturable virus, indicating a lack of infectiousness. Reference Bullard, Dust and Funk2,Reference Singanayagam, Patel and Charlett3 Additionally, as the prevalence of COVID-19 has declined due to widespread immunity from vaccination and previous infections, the positive predictive value of SARS-CoV-2 diagnostic testing among asymptomatic patients has also declined. Continued use of such testing in asymptomatic patients risks delays in care and unnecessary isolation due to clinical false-positive results. Reference Srinivasan, Gohil and Abeles5 Furthermore, the severity of SARS-CoV-2 infection has dramatically decreased as a result of vaccination and past infection. Reference Adjei, Hong and Molinari6 Fortunately, vaccination also appears to decrease the risk of long COVID Reference Azzolini, Levi and Sarti7 and the available medications (eg, paxlovid) decrease the risk of progression toward severe disease and long COVID. Reference Xie, Choi and Al-Aly8 These changes in both the epidemiology and the impact of SARS-CoV-2 infection have modified the public health paradigm from detecting and isolating every possible case to an approach aimed at preventing severe presentations. Along those lines, the Society for Healthcare Epidemiology of America (SHEA) has published a position statement recommending the suspension of asymptomatic diagnostic testing on hospital admission. Reference Talbot, Hayden and Yokoe9 Although it remains uncertain whether asymptomatic testing may still be warranted on admission to hospital units caring for patients at high-risk of severe disease (eg, transplant or behavioral health units), at the current stage of the pandemic, prevention of transmission of SARS-CoV-2 in healthcare facilities should capitalize on other horizontal infection prevention measures such as vaccination, testing and isolation of symptomatic patients, and improved ventilation.
This study had several limitations. These patients were from a single center. We used electronic data abstraction, which can be subject to missing data, particularly regarding the presence or absence of symptoms, as well as if patients were pre-symptomatic (ie, in the early phase of their illness) rather than asymptomatic throughout. Also, patients who had strand-specific RT-qPCR tests may have had unmeasured characteristics, making them less likely to be infectious. Because ordering the strand-specific RT-qPCR test was at the discretion of the treating provider, some bias toward the null may remain. Furthermore, there has been debate in the literature regarding the optimal Ct value that is clinically relevant and reflects infectiousness. Finally, we used the absence of viral replication as a proxy for infectiousness, although the strand assay has not been validated against gold standard viral culture. Reference Hogan, Huang and Sahoo4 The strengths of this study include the use of a novel method for evaluating SARS-CoV-2 infectiousness, a significant proportion of immunocompromised individuals (21%), and a long study period (August 2020–April 2022) that included multiple SARS-CoV-2 variant surges. Given the low rate of infectiousness among asymptomatic patients with a positive diagnostic SARS-CoV-2 test, our findings support the SHEA position statement to suspend the routine use of admission SARS-CoV-2 testing among asymptomatic patients. Reference Talbot, Hayden and Yokoe9,Reference Alsuhaibani, Kobayashi and Trannel10
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2023.210
Acknowledgements
Financial support
This work was partially supported by the National Institutes of Health (NIH grant no. R25 AI 147369).
Conflicts of interest
The authors have no conflicts of interest to disclose.




