To the Editor—Acinetobacter baumannii is a major pathogen related to several nosocomial infections, particularly ventilator-associated pneumonia. The worldwide emergence of carbapenem-resistant A. baumannii isolates (CRAB) constitutes a real threat owing to the few available therapeutic options.Reference Peleg, Seifert and Paterson 1 This resistance is most commonly mediated by carbapenemases, notably OXA-type carbapenemases but also metallo-β-lactamases.Reference Peleg, Seifert and Paterson 1
In 2007, several hospitals in Porto Alegre, the capital of the southernmost Brazilian state, presented CRAB outbreaks,Reference Martins, Kuchenbecker and Sukiennik 2 , Reference Martins, Kuchenbecker and Pilger 3 as did other Brazilian cities.Reference Schimith Bier, Luiz and Scheffer 4 After these first outbreaks, most Brazilian institutions remained with endemic rates of CRAB, including most hospitals of Porto Alegre.Reference Martins, Kuchenbecker and Pilger 3 In this study, we evaluated the molecular epidemiology of CRAB in 3 tertiary care hospitals in this city, 5 years after the outbreak, in order to assess whether clonal dissemination was, at least, one of the factors responsible for maintenance of high endemic rates of these isolates.
All Acinetobacter spp. isolates recovered from patients admitted at 3 tertiary care hospitals of Porto Alegre from March 1 through December 31, 2011, were included in the study.
Bacterial identification was performed by the Vitek 2 system (bioMérieux). The presence of bla OXA-51-like, bla OXA-23-like, bla OXA-24-like, bla OXA-58-like, and bla OXA-143 was evaluated by polymerase chain reaction assay using primers and a multiplex assay.Reference Higgins, Lehmann and Seifert 5 Minimum inhibitory concentrations (MICs) for cefepime, ciprofloxacin, ceftazidime, ampicillin/sulbactam, amikacin, polymyxin B, tigecycline, imipenem, and meropenem were determined by broth microdilution in CRAB isolates and interpreted according to Clinical and Laboratory Standards Institute guidelines 6 ; an MIC less than or equal to 2 mg/L was considered susceptibility to tigecycline. Pseudomonas aeruginosa ATCC 27853 was included as quality control in all tests. CRAB isolates were submitted to molecular typing by ApaI DNA macrorestriction followed by pulsed-field gel electrophoresis.Reference Seifert, Dolzani and Bressan 7 Results were interpreted by means of a dendrogram constructed using the band-based Dice coefficient method and, for the purposes of this study, isolates with at least 85% match were considered clones.
A total of 122 Acinetobacter spp. isolates were evaluated during the study period. Of these, 115 (94.3%) were identified as A. baumannii, owing to the presence of bla OXA-51-like gene. Eighty-four A. baumannii isolates (73.0%) were resistant to both carbapenems tested. They were recovered from respiratory secretions (59.5%), urine (15.4%), blood (11.9%), and other sites (14.1%). Among the 84 CRAB isolates, the MIC50 and MIC90 of both imipenem and meropenem were 32 and 64 mg/L, respectively (range, 16 to 256 mg/L). The MIC50 and MIC90 of polymyxin B were 0.25 and 0.5 mg/L, respectively (range, ≤0.125 to >64 mg/L); and 1 and 2 mg/L for tigecycline, respectively (range, ≤0.03 to 4 mg/L). Forty-eight isolates (41.7%) of the 115 isolates were resistant to all antimicrobials, except to polymyxin B and tigecycline. These extensively drug-resistant isolates were present in the 3 hospitals. Resistance to polymyxin B was identified in 4 (4.8%) of the 84 CRAB isolates (Table 1).
TABLE 1 MICs of the 4 Acinetobacter baumannii Isolates Resistant to Polymyxin B
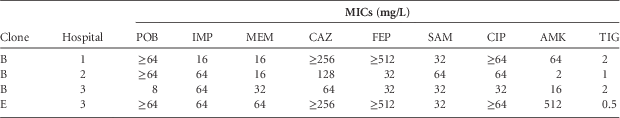
NOTE. AMK, amikacin; CAZ, ceftazidime; CIP, ciprofloxacin; FEP, cefepime; IMP, imipenem; MEM, meropenem; MIC, minimum inhibitory concentration; POB, polymyxin B; SAM, ampicillin-sulbactam; TIG, tigecyclin.
The presence of bla OXA-23-like gene was detected in all CRAB isolates and no other gene was detected in any isolate. Molecular typing revealed that the isolates were clustered into 7 clones (A to G). Clones A (33.7%), B (31.2%), and C (15.0%) represented 80% of all isolates. The remaining 20% belonged to the other 4 clones at similar proportions. A, B, and D were found at the 3 hospitals evaluated. Polymyxin B–resistant strains belonged to 2 different pulsed-field gel electrophoresis clones (Table 1).
CRAB outbreaks have been observed since the early 1990s.Reference Go, Urban and Burns 8 However, in Brazil the first CRAB outbreak was reported only in 2003.Reference Martins, Kuchenbecker and Sukiennik 2 Later on, in 2007, a wide CRAB outbreak occurred in our city, Porto Alegre. Since then, endemic rates of CRAB have been observed. Our study showed a high prevalence of OXA-23–mediated carbapenem resistance among A. baumannii isolates 5 years after the outbreak. Notably, when compared with the isolates investigated during the outbreak in 2007,Reference Martins, Kuchenbecker and Sukiennik 2 , Reference Martins, Kuchenbecker and Pilger 3 it was observed that the same clones remained in the city hospitals (data not shown). This fact indicates that horizontal transmission has a major role in the maintenance of these high rates of carbapenem resistance among our institutions. It also suggests that specific clones may be better adapted to the nosocomial environment, resulting in a “homogeneous” persistence of specific CRAB strains. All CRAB isolates were positive for bla OXA-23-like gene and negative for all the other OXA-encoding genes investigated, including that encoding OXA-143, which is highly frequent in the southeastern region of the country.Reference Mostachio, Levin and Rizek 9
The prevalence of polymyxin B resistance among CRAB strains may still be considered low, even though these few isolates presented resistance to virtually all other classes with the few exceptions of amikacin for 1 isolate and tigecycline for all.
Our study was limited by the lack of multilocus sequence typing analysis, which would contribute to the knowledge of the molecular epidemiology of CRAB isolates. Although many distinct sequence types of CRAB, including some international clones, have been identified in Brazil,Reference Martins, Dalla-Costa and Uehara 10 , Reference Chagas, Carvalho and de Oliveira Santos 11 there still are no data from this Brazilian region.
In summary, we demonstrated the persistence of a few clones responsible for endemic levels of CRAB isolates in hospitals in a Brazilian city. Notably, 3 of 7 clones remained as the major strains at least 5 years after an initial outbreak in this city. These findings challenged the effectiveness of infection control measures to control the dissemination of CRAB after an initial large outbreak.
Acknowledgments
Financial support. CAPES Foundation, Ministry of Education of Brazil, Brasília, Brazil; FIPE/HCPA (Research and Events Support Fund at Hospital de Clinicas de Porto Alegre); CNPq, Ministry of Science and Technology, Brazil (research fellowship 305263/2011–0 to A.P.Z.).
Potential conflicts of interest. All authors report no conflicts of interest relevant to this article.



