Introduction to the Executive Summary
This document summarizes the recommendations that are included within each section of “A Compendium of Strategies to Prevent Healthcare-Associated Infections in Acute-Care Hospitals: 2022 Updates.” Reference Buetti, Marschall and Drees1–Reference Patel, Advani and Kofman8
Appendix Tables 1 and 2 describe the criteria used to determine the quality of evidence ratings and classification of recommendations as essential practices versus additional approaches. These criteria are discussed in more detail in the “Introduction to a Compendium of Strategies to Prevent Healthcare-Associated Infections in Acute-Care Hospitals: 2022 Updates,” Reference Yokoe, Maragakis and Advani9 which also provides a summary of the background and the methods used to develop the Compendium: 2022 Updates. The individual Compendium 2022 sections Reference Buetti, Marschall and Drees1–Reference Patel, Advani and Kofman8 summarize the major changes to the recommendations from the Compendium: 2014 Updates. Reference Yokoe, Anderson and Berenholtz10
Appendix Table 3 lists the authors and members of the Advisory Group and Expert Panel for the Compendium: 2022 Updates.
Executive summary
Strategies to prevent catheter-associated urinary tract infections (CAUTIs)

Strategies to prevent central-line–associated bloodstream infections (CLABSIs)

Strategies to prevent Clostridioides difficile infections (CDIs)

Strategies to prevent methicillin-resistant Staphylococcus aureus (MRSA) transmission and infection
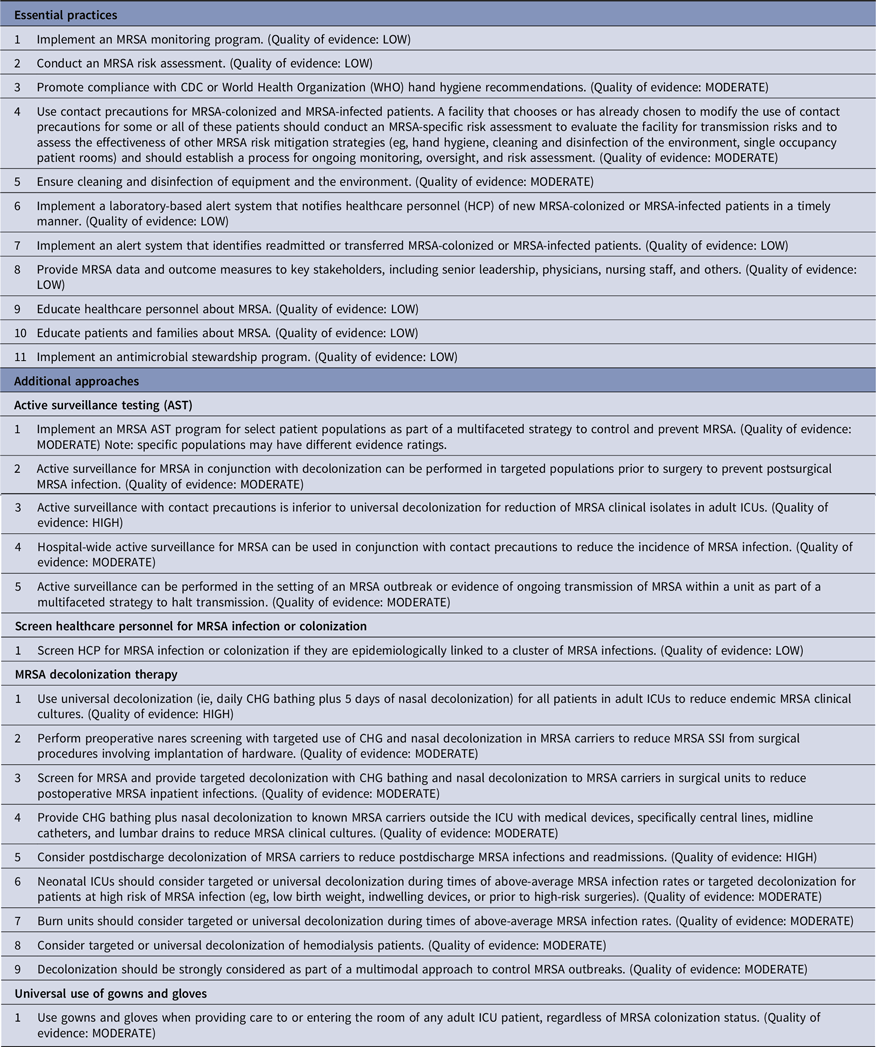
Strategies to prevent surgical-site infections (SSIs)
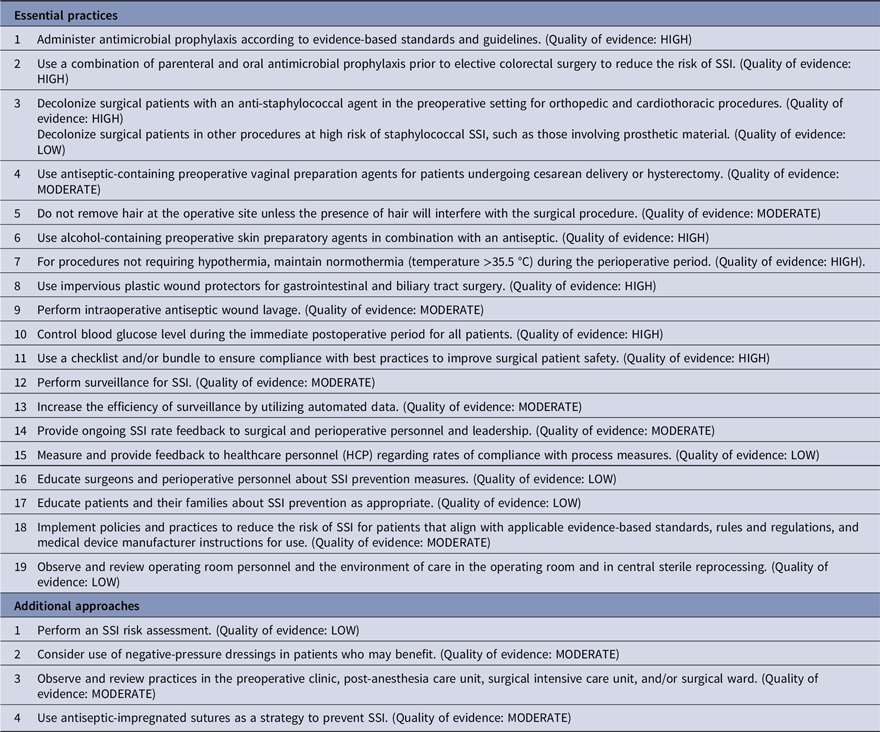
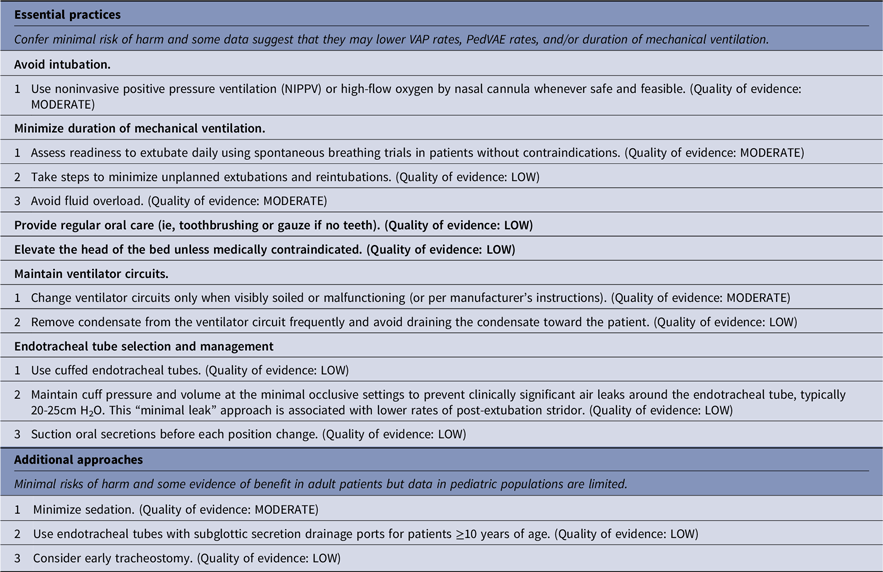
Strategies to prevent ventilator-associated pneumonia (VAP) and ventilator-associated events (VAEs)
Adult patients

Preterm neonatal patients

Pediatric patients
Strategies to prevent nonventilator hospital-acquired pneumonia (NV-HAP)

Strategies to prevent healthcare-associated infections through hand hygiene

Implementing strategies to prevent healthcare-associated infections
Standard approach to implementation

Examples of implementation frameworks

Acknowledgments
The Compendium Partners thank the authors for their dedication to this work, including maintaining adherence to the rigorous process for the development of the Compendium: 2022 Updates, involving but not limited to screening of thousands of articles; achieving multilevel consensus; and consideration of, response to, and incorporation of many organizations’ feedback and comments. We acknowledge these efforts especially because they occurred as the authors handled the demands of the COVID-19 pandemic. The authors thank Valerie Deloney, MBA, for her organizational expertise in the development of this manuscript and Janet Waters, MLS, BSN, RN, for her expertise in developing the strategies used for the literature searches that informed this manuscript. The authors thank the many individuals and organizations who gave their time and expertise to review and provide comments on the Compendium: 2022 Updates, including 2018–2023 members of the SHEA Guidelines Committee, the SHEA Publications Committee, which helped oversee the planning and development of the Compendium: 2022 Updates, and the SHEA Board of Trustees; the IDSA Standards and Practice Guidelines Committee and the IDSA Board of Directors; members of the National Foundation of Infectious Diseases (NFID) Sara E. Cosgrove, MD, MS (MRSA, CAUTI, SSI, CDI), Kathleen H. Harriman, PhD, MPH, RN (MRSA), S. Shaefer Spires, MD (MRSA, CAUTI, SSI, CDI); members of the Society of Infectious Diseases Pharmacists (SIDP) Emily Heil, MD (VAP/VAE/NV-HAP), Kayla R. Stover Hielscher, PharmD (VAP/VAE/NV-HAP), David A. Cretella, PharmD (VAP/VAE/NV-HAP), Tracy Zembles, PharmD (Implementation), Mary Joyce B. Wingler, PharmD (Implementation), Jarrett Amsden, PharmD (Implementation), and Alan E. Gross, PharmD; and members of the Surgical Infection Society (SIS) Joseph Cuschieri, MD, Rondi B. Gelbard, MD, Sabrina D. Goddard, MD, George E. Koch, MD, and Sebastian D. Schubl, MD. The Compendium Partners and authors thank everyone who contributed and whose input informed and improved the Compendium: 2022 Updates. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the US Department of Veterans’ Affairs.
Financial support
Financial support for the Compendium: 2022 Updates was provided by the Society for Healthcare Epidemiology of America (SHEA).
Conflict of interest disclosure
The following disclosures reflect what has been reported to SHEA. To provide thorough transparency, SHEA requires full disclosure of all relationships, regardless of relevancy to the topic. Such relationships as potential conflicts of interest are evaluated in a review process that includes assessment by the SHEA Conflict of Interest Committee and may include the Board of Trustees and Editor of Infection Control and Hospital Epidemiology. The assessment of disclosed relationships for possible conflicts of interest has been based on the relative weight of the financial relationship (ie, monetary amount) and the relevance of the relationship (ie, the degree to which an association might reasonably be interpreted by an independent observer as related to the topic or recommendation of consideration). Potential conflicts have been disclosed by the Compendium: 2022 Updates authors as follows:
CAUTI section: S.A. reports consulting fees with IDSA, bioMerieux, Locus Biosciences, and GSK, and participation on a bioMerieux Advisory Board, and is co-owner of IPEC Experts, LLC. J.M. reports grant funding from the AHRQ, the CDC, the Ralph E. Wilson Foundation, and the VA HSR&D. All other authors report no conflicts of interest related to this article.
CDI section: L.K.K. reported a research grant from Merck. R.C. reported an advisory role with Moderna, Novavax, and Pfizer (consultant, speakers bureau, research contract), and Sanofi (speakers bureau). C.J.D. reported a research grants from Clorox and Ecolab. G.D. reported a research grant from Pfizer. V.G.L. reported a consultant role with Ferring. D.J.W. reported advisory roles for Merck, Pfizer, PDI, Wellair, GAMA, and Germitec, and serving as a data safety monitoring board member at GSK. E.R.D. reported advisory roles for Merck, Pfizer, Seres, Ferring, Abbott, Summit, GSK, and research grants from Pfizer, Theriva Biologics, and Ferring. All other authors report no conflicts of interest related to this article.
CLABSI section: N.B. received Mobility grants from the Swiss National Science Foundation (grant nos. P400PM_183865 and P4P4PM_194449) and a grant from the Bangerter-Rhyner Foundation. J.M. is the recipient of a project grant on surgical-site infections from the Swiss National Science Foundation (grant no. 32003B_179500, “Understanding the drivers of surgical site infection: Investigating and modeling the Swissnoso surveillance data”). L.M. served as an advisor and consultant to Marvao Medical Devices, Pristine Access Technologies, and Citius Pharma. L.H. served as an advisor and consultant to B Braun Medical, BD Medical, Atrion Medical, Nexus Medical, Teleflex. M.E.R. served as an advisor and consultant to 3M, Becton Dickinson, and Cetius, and Teleflex, and received honoraria from Teleflex. All other authors report no conflicts of interest related to this article.
Hand hygiene section: A.A. reported payment from the analysis group for an expert presentation on litigation for prevention of infection in the community setting. K.H. reported consulting for Medillum and Nozin, a grant from the NC Department of Public Health, and providing a lecture for PDI. R.O. reported consulting for Specified Technologies and Northshore University Health System, providing expert testimony for Saxton & Stump, providing a lecture for the Teleflex Medical Advisory Board, providing subject-matter expertise for APIC for AHA HRET, and performing peer review of a manuscript for Joint Commission Resources. All other authors report no conflicts of interest related to this article.
MRSA section: A.M. received a research grant from Merck. A.H. served as an advisory and consultant for Cubist, UpToDate, and Premier, Inc. S.H. led a clinical trial in which participating hospitals received contributed product from Sage Products, Inc. J.M. conducted a clinical trial in which participating hospitals received contributed product from Sage Products, Inc. All other authors report no conflicts of interest related to this article.
SSI section: D.J.A. is the owner of Infection Control Education for Major Sports, LLC, has grants from the CDC and the AHRQ and has received royalties for authorship on UpToDate. A.C.N. is the Data and Safety Monitoring Board Chair for the CAV-AVI Neonatal PK Study with Pfizer. All other authors report no conflicts of interest related to this article.
VAP, VAE, NV-HAP section: M.K. has received grant funding from CDC and AHRQ plus royalties from UpToDate, Inc. R.B. has served as an advisor and consultant to Pfizer, Mallinckrodt, Ventec Life Systems, Vyaire and Zoll Medical. K.A.C. has received honoraria from BD, CloroxPro, and Elsevier. L.R.G. has received honoraria from Premier, Inc, Care Fusion, Infection Control Today, and Advanced Sterilization Products. All other authors report no conflicts of interest related to this article.
Implementation section: K.K.T. is the owner of the consulting company Trivedi Consults, LLC. V.M.D. is the owner of the consulting company Youngtree Communications, LLC. R.C. has consulting relationships with Moderna, Novavax, and Pfizer (speakers bureau, research contract) and Sanofi (speakers bureau). M.L.S. has grant funding from 3M and PDI for nasal decolonization. All other authors report no conflicts of interest related to this article.
Expert Panel: H.M.B. reports institutional funding from CDC for a Prevention Epicenter; K.A.B. is the PI for multicenter clinical trials funded for institution by Pfizer, Gilead, and Enanta; M.K.H. reports institutional funding from CDC for a Prevention Epicenter and from the Chicago Department of Public Health for a Regional Innovative Public Health Laboratory; R.A.W. is a paid reviewer for UpToDate. All other authors report no conflicts of interest related to this article.
Appendix
Table 1. Quality of Evidence

Based on the CDC Healthcare Infection Control Practices Advisory Committee (HICPAC) “Update to the Centers for Disease Control and Prevention and the Healthcare Infection Control Practices Advisory Committee Recommendations Categorization Scheme for Infection Control and Prevention Guideline Recommendations” (October 2019), 34 the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE), Reference Guyatt, Oxman and Vist35 and the Canadian Task Force on Preventive Health Care. 36
Table 2. Level of Recommendation

Table 3. Compendium Leadership and Authors







