One limitation of current cleaning and disinfection strategies is that disinfected surfaces rapidly become recontaminated. Reference Donskey1,Reference Weber, Rutala, Sickbert-Bennett, Kanamori and Anderson2 This limitation has led to interest in the development of technologies that provide continuous decontamination between episodes of manual cleaning. Reference Weber, Rutala, Sickbert-Bennett, Kanamori and Anderson2 One promising technology is continuously active quaternary ammonium disinfectants that contain polymer coatings that bind to surfaces resulting in persistent antimicrobial activity. Reference Weber, Rutala, Sickbert-Bennett, Kanamori and Anderson2 Rutala et al Reference Rutala, Gergen, Sickbert-Bennett, Anderson and Weber3 reported that a continuously active quaternary ammonium disinfectant demonstrated sustained antimicrobial activity against several pathogens after 24 hours. Others have reported reductions in contamination of surfaces treated with continuously active quaternary ammonium disinfectants, and in 1 study, healthcare-associated infections were reduced. Reference Schmidt, Fairey and Attaway4–Reference Ellingson, Pogreba-Brown, Gerba and Elliott6
One potential application of continuously active disinfectants is portable medical equipment. Portable devices are often contaminated and have been implicated as a potential vector for transmission. Reference Donskey1 Current guidelines recommend that medical equipment that comes into contact with intact skin be cleaned and decontaminated after each patient use. Reference Donskey1 However, cleaning and disinfection of portable equipment is often suboptimal. Reference Donskey1,Reference Suwantarat, Supple, Cadnum, Sankar and Donskey7 We hypothesized that application of a continuously active disinfectant would be effective in reducing contamination of medical equipment used in a hospital setting.
Methods
Study setting
The Cleveland VA Medical Center is a 215-bed hospital with an affiliated 250-bed long-term care facility (LTCF). Portable medical equipment is stored between use in common areas on each ward. According to hospital policies, equipment should be decontaminated with antimicrobial wipes by the provider after use, but compliance with this policy is not monitored. Reference Suwantarat, Supple, Cadnum, Sankar and Donskey7
Evaluation of persistent antimicrobial activity of the continuously active disinfectant
Sani-24 germicidal spray is Environmental Protection Agency (EPA)-registered as Firebird F130 (Microban Products, Huntersville, NC) and marketed by Professional Disposables International as Sani-24. The product has a 24-hour residual disinfectant claim. Reference Rutala, Gergen, Sickbert-Bennett, Anderson and Weber3 We used EPA protocol #01-1A to assess persistent activity against methicillin-resistant Staphylococcus aureus (MRSA, clinical strain), vancomycin-resistant Enterococcus faecium (VRE, VanB-type strain), carbapenem-resistant strains of Klebsiella pneumoniae (American Type Culture Collection [ATCC] BAA-1705) and Enterobacter aerogenes (clinical strain), Candida auris (Centers for Disease Control and Prevention strain 0381), and Clostridioides difficile spores (ATCC 43598). 8 The method requires the use of a standardized abrasion machine to apply multiple wet and dry wiping steps over 24 hours in addition to multiple reinoculations of the pathogen. Reference Rutala, Gergen, Sickbert-Bennett, Anderson and Weber3,8 After 24 hours, the test surface was inoculated with 106 colony-forming units (CFU) of the test pathogen and the log10 reduction after 5 minutes was calculated. Reference Rutala, Gergen, Sickbert-Bennett, Anderson and Weber3,8 A standard quaternary ammonium-alcohol disinfectant spray (Cavicide 1 SKU 13-5024) was used for comparison. The test surface was glass slide carriers with 5% fetal calf serum as the organic load. The neutralizer was 1.5% lecithin and 5% Tween 80.
Evaluation of effectiveness in reducing contamination of portable medical equipment
The evaluation was conducted on wards in the hospital and the LTCF. In total, 114 portable devices were assigned by block randomization to receive no treatment (N = 38), a single spray application of Cavicide quaternary ammonium-alcohol disinfectant (N = 38), or a single spray application of the continuously active disinfectant (N = 38). The devices were sprayed once with adequate product to thoroughly wet the surfaces and allowed to air dry. The equipment included portable vital signs equipment (N = 40), bladder scanners (N = 19), electrocardiogram machines (N = 25), work stations on wheels (N = 17), doppler ultrasounds (N = 5), portable scales (N = 6), an infusion pump (N = 1), and a vein finder (N = 1).
The devices were sampled at baseline and 1, 4, and 7 days after treatment using CultureSwabs (Becton Dickinson, Franklin Lakes, NJ) premoistened with Dey-Engley neutralizer (Remel Products, Lenexa, KS). Prespecified sites were sampled focusing on frequently touched areas; given the variability in devices, the surface area sampled varied. Equipment that could not be located during days 1, 4, or 7 was excluded from analysis. The swabs were processed for total aerobic colony counts, S. aureus, and enterococci. A previous study demonstrated that S. aureus and enterococci were frequently recovered from equipment. Reference Suwantarat, Supple, Cadnum, Sankar and Donskey7 Use and cleaning of the equipment were not monitored.
Data analysis
Analysis of variance (ANOVA) with a Tukey honest significance test was used to compare mean aerobic colony counts (CFU) on days 1–7 to baseline levels. A logistic model was used to compare the frequency of contamination with S. aureus and/or enterococci on days 1–7. All analyses were performed using R version 3.5.1 statistical software (The R Foundation for Statistical Computing, Vienna, Austria).
Results
In laboratory testing, the continuously active quaternary ammonium disinfectant resulted in ≥5 log10 reduction of the MRSA, VRE, C. auris, and carbapenem-resistant K. pneumoniae and E. aerogenes test strains with 5 minutes of exposure but no reduction in C. difficile spores. The standard quaternary ammonium disinfectant resulted in ≤0.5 log10 reduction of the test strains.
The continuously active disinfectant resulted in sustained significant reductions in aerobic colony counts on equipment in comparison to the baseline level of contamination (P < .001) (Fig. 1). The standard quaternary ammonium disinfectant did not result in an overall reduction in aerobic colony counts (P = .09). There was no reduction in aerobic colony counts for the untreated control equipment.
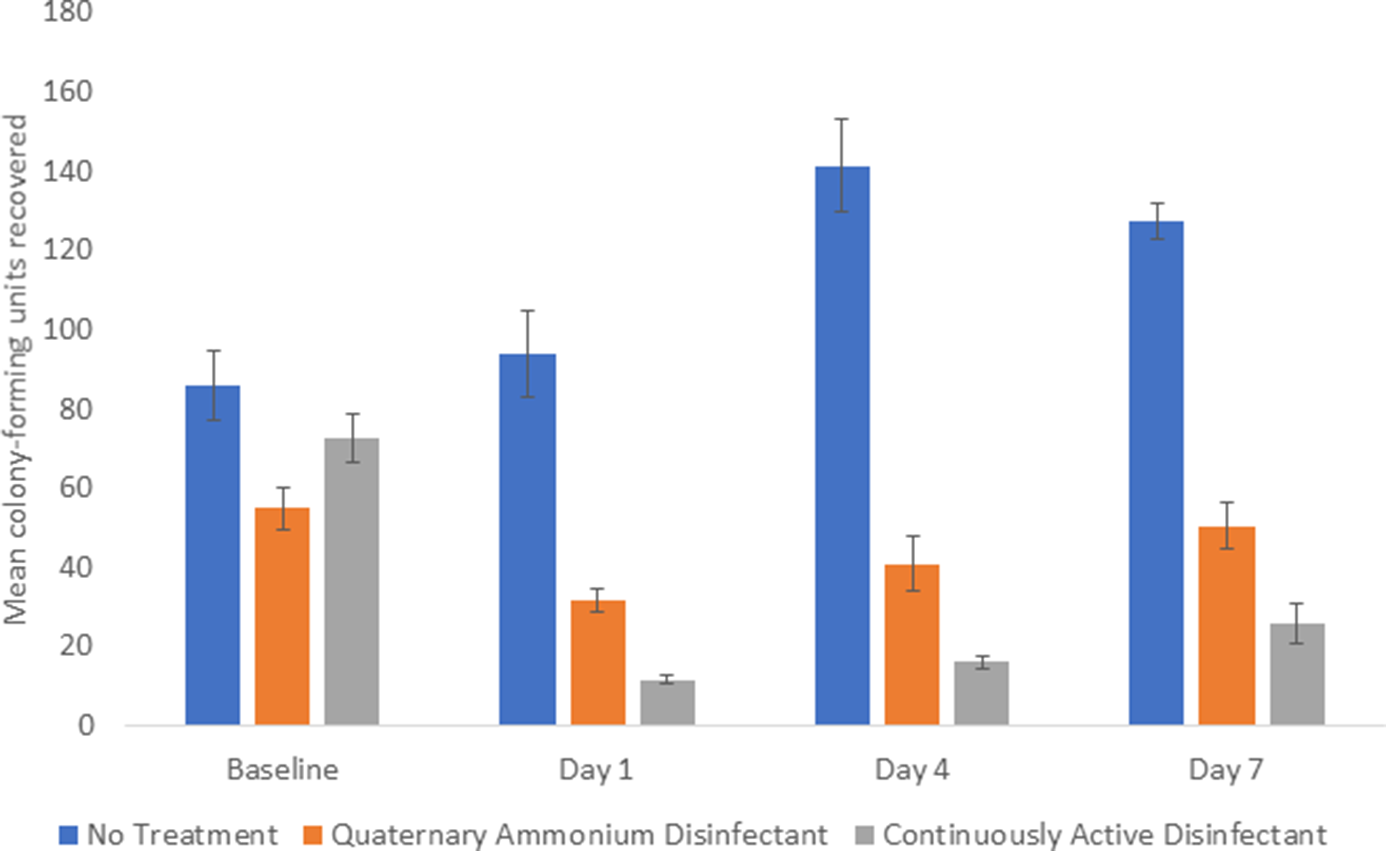
Fig. 1. Comparison of total aerobic colony-forming units (CFU) recovered from portable medical equipment at baseline and 1, 4, and 7 days after no treatment (controls) or treatment with a continuously active quaternary ammonium disinfectant or a standard quaternary ammonium disinfectant with no claim for residual antimicrobial activity.
The percentage of sites positive for S. aureus and/or enterococci was significantly reduced on days 1–7 in the continuously active disinfectant group (3 of 93, 3%) versus both the no treatment group (20 of 97, 21%; P < .001) and the quaternary ammonium disinfectant group (11 of 97, 11%; P = .048) (Fig. 2).
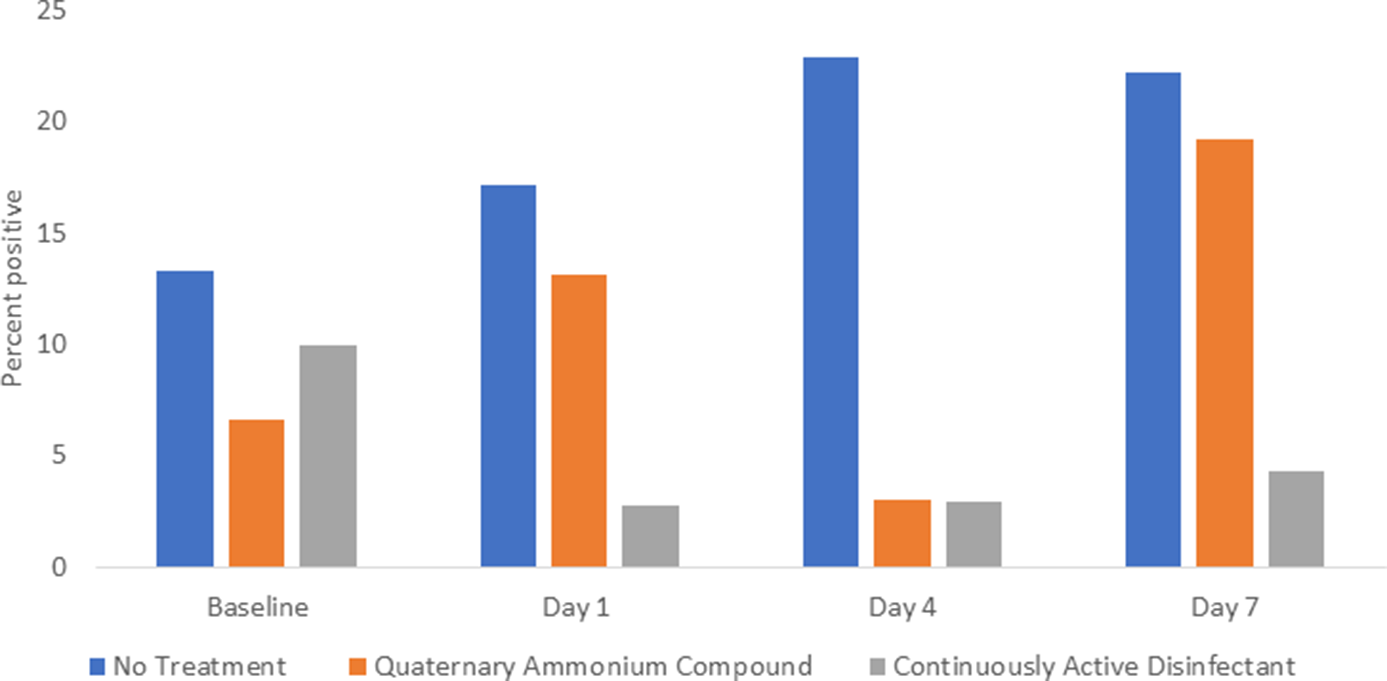
Fig. 2. Comparison of Staphylococcus aureus and enterococci recovered from portable medical equipment at baseline and 1, 4, and 7 days after no treatment (controls) or treatment with a continuously active quaternary ammonium disinfectant or a standard quaternary ammonium disinfectant with no claim for residual antimicrobial activity.
Discussion
We found that a continuously active disinfectant provided sustained antimicrobial activity against multiple bacterial pathogens and C. auris. On hospital and LTCF wards, a single spray application of a continuously active disinfectant on portable medical equipment resulted in sustained reductions in aerobic colony counts over 7 days and reduced recovery of S. aureus and enterococci. These results suggest that application of the continuously active disinfectant could be useful to reduce the risk for transmission of pathogens by portable devices.
Our findings are consistent with a recent report from Rutala et al Reference Rutala, Gergen, Sickbert-Bennett, Anderson and Weber3 in which the same continuously active disinfectant provided sustained antimicrobial activity against bacterial pathogens and C. auris. Rutala et al Reference Rutala, Gergen, Sickbert-Bennett, Anderson and Weber3 reported only ∼2 log10 reduction in carbapenem-resistant Enterobacter spp and K. pneumoniae, whereas we found >5 log10 reductions in these organisms. This difference could potentially be related to differences in test surfaces or other methodologic differences.
Our results suggest that portable medical equipment could be a good place to apply continuously active disinfectant products. Portable devices often become contaminated during medical procedures and patient care activities and are infrequently cleaned. Reference Alhmidi, Cadnum and Koganti9,Reference Alhmidi, Cadnum and Koganti10 Our data suggest that intermittent application of a continuously active disinfectant could provide sustained antimicrobial activity against bacteria and Candida spp.
Our study has several limitations. We did not monitor use and cleaning of the equipment, and we only collected cultures over 7 days. We assessed the impact of the product on S. aureus and enterococci. Additional studies are needed to assess additional pathogens. Although the product demonstrated sustained activity despite multiple cycles of dry and wet wiping, it can be removed by chlorine, hydrogen peroxide, and detergents. Reference Rutala, Gergen, Sickbert-Bennett, Anderson and Weber3 Therefore, repeated application would be required in settings where equipment is cleaned with other disinfectants or detergents. Use of the product would not eliminate the need for thorough cleaning prior to application.
In conclusion, application of continuously active quaternary ammonium disinfectants could be useful to reduce contamination of medical equipment. Future studies are needed to determine the impact of these products on colonization and infection with healthcare-associated pathogens.
Acknowledgments
We thank the nursing service personnel at the Cleveland VA Medical Center for their assistance in the study.
Financial support
This work was supported by a grant from PDI to C.J.D. and by a Merit Review grant (grant no. CX001848) from the Department of Veterans’ Affairs to C.J.D. PDI provided input on the study design but did not contribute to data analysis or interpretation and did not aid in writing or editing the manuscript.
Conflicts of interest
C.J.D. has received research grants from PDI, Clorox, and Pfizer. All other authors report no conflicts of interest relevant to this article.





