Carbapenemase-producing bacteria (CPB) are rapidly emerging worldwide but are still infrequent in Switzerland. Since 2013, ANRESIS (the Swiss Center for Antibiotic Resistance) has reported increasing numbers of CPB; cases have more than doubled from 250 reported CPB isolates in 2013 to 542 cases in 2021. 1 The main CPB species are Klebsiella pneumoniae (26%) and Enterobacter spp (18%), whereas the most frequently detected carbapenemases are oxacillinase (OXA) genotypes, followed by K. pneumoniae carbapenemases (KPC). 1,Reference Ramette, Gasser and Nordmann2 Travel or migration from countries with higher endemicity is still considered the main pathway of introduction into regions of low endemicity where CPB are mainly recovered in the context of healthcare-acquired infections. Reference Ramette, Gasser and Nordmann2,Reference Khawaja, Kirveskari and Johansson3 Duration of CPB carriage is usually unknown and spontaneous decolonization is frequent, yet CPB carriage might persist up to several months to years, Reference Vink, Otter and Edgeworth4 with higher risk of prolonged carriage in immunocompromised patients. Reference Jimenez, Fennie and Munoz-Price5 Limited treatment options for infections caused by CPB represent a major challenge for healthcare systems, possibly increasing morbidity, mortality, and healthcare costs. Reference Vink, Otter and Edgeworth4,Reference Stewardson, Marimuthu and Sengupta6
In contrast, extended-spectrum β-lactamase–producing Enterobacterales (ESBL-PE) have reached endemic levels in the Swiss population. 1 Predictive patient-related variables have been described for patients harboring CPB and ESBL-PE, respectively. These analyses indicated similar exposures and risk factors, such as travel to areas of high endemicity, recent hospitalization, comorbidities, invasive procedures, or previous antibiotic therapy. All of these factors impede decision making for patients at risk of CPB carriage. Reference Khawaja, Kirveskari and Johansson3,Reference Segagni Lusignani, Presterl, Zatorska, Van den Nest and Diab-Elschahawi7–Reference Reuland, Al Naiemi and Kaiser12
We analyzed characteristics of patients colonized with CPB in a tertiary-care center in a setting of low endemicity. We compared patient-related risk factors between patients colonized with CPB and patients colonized with ESBL-PE to gain further insights into the epidemiology of these high-priority antibiotic-resistant pathogens and to facilitate early detection of patients at risk. These insights will help guide empirical antibiotic treatment and correct implementation of pre-emptive infection control measures.
Methods
Setting and participants
This retrospective cohort study was conducted at the University Hospital Basel (USB), Switzerland, an academic tertiary-care center. The microbiology laboratory at USB identified patients harboring CPB between January 2008 and July 2019. Eligibility criteria were age ≥18 years, hospitalization in the USB and detection of at least 1 species of CPB from any sample within the respective hospitalization. The institutional infection prevention and control guidelines require all patients admitted directly from acute-care settings abroad or with a history of CPB colonization to be screened on admission. In addition, colonization with ESBL-PE and/or CPB is screened for inpatients requiring at least 24 hours of intensive care or admitted to the hematological transplant unit. A cohort of patients with ESBL-PE detected between January 2016 and December 2018 was used for comparisons of risk factors and exposures between patients colonized with CPB and ESBL-PE. Hospitalized patients aged ≥18 years with detection of ESBL-PE from any sample within the corresponding hospitalization, identified by the microbiology laboratory, were included in the ESBL-PE group. Data regarding patient selection and characteristics of the ESBL-PE cohort were previously published in the ESBL Infect study. Reference Vock, Aguilar-Bultet, Egli, Tamma and Tschudin-Sutter13 In both cohorts, patients were excluded if refusal of subsequent use of their data was documented using the general informed consent tool of the USB.
This study was approved by the local ethics committee (Ethikkomission Nordwest- und Zentralschweiz (EKNZ), project-IDs 2019-01548 and 2017-00100). We adhered to the “Strengthening the Reporting of Observational Studies in Epidemiology” guidelines. Reference von Elm, Altman and Egger14
Study data collection and definitions
Patient data were collected from electronic medical records, anonymized, and entered into a secure REDCap (Research Electronic Data Capture) database. Reference Harris, Taylor, Thielke, Payne, Gonzalez and Conde15,Reference Harris, Taylor and Minor16 Data included (1) demographics; (2) clinical characteristics such as comorbidities, vascular hardware or urinary catheterization, recent surgeries, active open wounds, history of allogeneic stem-cell or solid-organ transplantation; (3) current treatment (concomitant medication, antibiotic therapy, immunosuppressive therapy); and (4) microbiological data. Definitions are provided in the Supplementary Material (online).
Microbiological analyses
Both CPB and ESBL-PE were identified using standard diagnostic approaches. Identification methods of ESBL-PE were previously described in the ESBL-Infect study. Reference Vock, Aguilar-Bultet, Egli, Tamma and Tschudin-Sutter13 Testing for CPB changed during the study period. In principal, phenotypic testing was increasingly replaced with genotypic testing according to the increasing availability of respective assays. Most recently, screening was performed using chrom ID Carba Smart agar (bioMérieux, Marcy-l’Étoile, France). After species identification, Enterobacterales were tested for carbapememase genes using the eazyplex Superbug CRE panel (easyplex, Gars-Bahnhof, Germany) detecting KPC, VIM, NDM, OXA-48, and OXA-181. If phenotypic testing suggests carbapenem resistance and genotypic screening is negative, further phenotypic testing is performed using the Mastdiscs combi Carba plus test (Mast Group, Reinfeld, Germany). Pseudomonas spp were analyzed with GeneXpert Carba-R (Cepheid Switzerland, Thalwil, Switzerland) detecting VIM, IMP-1, NDM, KPC, and OXA-48. Acinetobacter baumannii–producing carbapenemases were identified using the eazyplex SuperBug complete A test system, which includes KPC, VIM, OXA-48, OXA-23, OXA-40, and OXA-58.
Statistical analyses
Sample size estimation was fixed based on identification of eligible patients by the microbiology laboratory within the study periods. Missing data were considered as the absence of an assessed factor. Descriptive analyses were summarized as counts and proportions for categorical variables, using the Fisher's exact test for comparisons. Medians and interquartile ranges of continuous variables were analyzed using the Mann-Whitney U test, as the Shapiro-Wilk revealed all continuous variables to be abnormally distributed. Univariable and multivariable regression analyses were applied for comparisons of the CPB group versus the ESBL-PE group as well as subgroup analyses of the CPB group. The Hosmer-Lemeshow goodness-of-fit test was applied to assess model fit (insignificant P values indicated adequate fit). We performed sensitivity analyses (1) excluding patients in the CPB group with nonfermenters and (2) excluding patients in the CPB group with nonfermenters and Enterobacterales not recovered during the same timeframe as patients colonized with ESBL-PE. Statistical significance was set at P < .05. Statistical analyses were performed using STATA version 16.1 software (StataCorp, College Station, TX).
Results
Within the study period, inclusion criteria were met by 50 patients in the CPB group and by 572 patients in the ESBL-PE group. The baseline characteristics, exposures and microbiological data of both the CPB and the ESBL-PE cohorts are presented in Table 1 and Supplementary Table 1 (online).
Table 1. Baseline Characteristics of the CPB Group and the ESBL-PE Group

Note. CPB, carbapenemase-producing bacteria; ESBL-PE, extended-spectrum β-lactamase–producing Enterobacterales; IQR, interquartile range; ICU, intensive care unit; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci. Bold indicates statistical significance.
a Within the previous 12 mo.
b Within 30 d prior to CPB detection.
c To be in place for at least 7 d prior to CPB detection.
d refers to ESBL-PE and/or CPB in the CPB group and to only ESBL-PE in the ESBL-PE group.
e Within 3 mo prior to CPB detection.
CPB group
Overall, 42 patients (84.0%) had a history of hospitalization within the previous 12 months. Among 31 patients (62.0%) travelling outside Switzerland within the prior 12 months, 30 patients had been hospitalized abroad. For 6 patients (12.0%) neither exposure to healthcare settings nor travel abroad was recorded. Antibiotic therapy within 3 months prior to the index hospitalization was administered in 39 patients (78.0%). Among 33 patients (66.0%) screened for multidrug-resistant bacteria at hospital admission, co-colonization with ESBL-PE was detected in 6 patients. First detection of CPB within the hospitalization derived mainly from clinical samples (26 patients, 52.0%), followed by admission screening samples (16 patients, 32.0%) and consecutive screening samples (8 patients, 16.0%). This screening was performed routinely in high-risk wards such as the intensive care unit (ICU) and the hematologic ward. In patients with a history of hospitalization abroad, first detection of CPB derived from admission screenings in 10 cases (33.3%), from consecutive screenings in 4 cases (13.3%), and from clinical samples in 16 cases (53.3%).
ESBL-PE group
Travel history outside Switzerland was positive in 15.6% of patients, and 48.8% had received antibiotic medication in the prior 3 months.
Subgroup analyses of CPB patients according to travel history
A subgroup analysis comparing patients of the CPB group with (n = 31) versus without (n = 19) travel history was performed (Supplementary Table 2 online). Admission from other acute-care facilities (OR, 3.88; 95% CI, 1.12–13.47; P = .033) and a history of hospitalization (OR, 6.69; 95% CI, 1.19–37.71; P = .031) were associated with the travel subgroup. A history of CPB/ESBL-PE colonization (OR, 0.20; 95% CI, 0.05–0.82; P = .025) and recent immunosuppressive therapy (OR, 0.18; 95% CI, 0.04–0.83; P = .028) were associated with the CPB nontraveling subgroup. Microbiological differences of CPB species and carbapenemases genotypes between CPB travel versus nontravel subgroups are shown in Supplementary Table 3 (online). Acinetobacter baumannii and OXA-type carbapenemases were more frequently recovered in patients with a travel history than without.
Table 2. Comparison of Risk Factors Between Patients With Detection of CPB Versus Patients With Detection of ESBL-PE
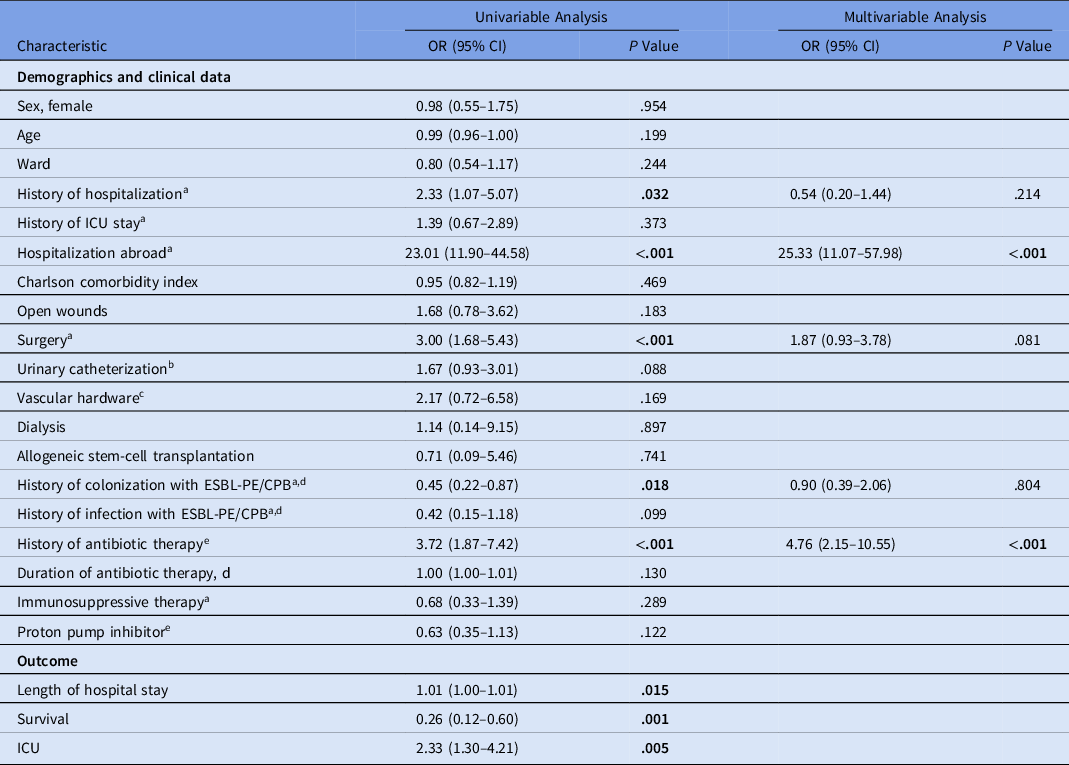
Note. CPB, carbapenemase-producing bacteria; ESBL-PE, extended-spectrum β-lactamase–producing Enterobacterales; OR, odds ratio; CI, confidence interval, ICU, intensive care unit. Bold indicates statistical significance.
a Within the past 12 mo.
b Within 30 d prior to CPB detection.
c To be in place for at least 7 d prior to CPB detection.
d Refers to ESBL-PE and/or CPB in the CPB group and to only ESBL-PE in the ESBL-PE group.
e Within 3 mo prior to CPB detection.
Comparison of the CPB group with the ESBL-PE group
Hospitalization abroad, recent surgery, and history of antibiotic therapy within the prior 3 months were associated with carriage of CPB in univariable analysis. History of colonization with CPB and/or ESBL-PE was associated with detection of ESBL-PE. After multivariable analysis, hospitalization abroad and history of antibiotic therapy remained associated with detection of CPB (Hosmer Lemeshow χ2, 5.65; P = .582) (Table 2).
Detection of CPB was associated with a longer duration of hospital stay and a lower survival rate (Table 2).
Subgroup analyses of the CPB cohort versus patients of the ESBL-PE cohort colonized with only E. coli and only K. pneumoniae were performed additionally.
Multivariable analyses of the CPB group versus the ESBL E. coli group revealed hospitalization abroad and history of antibiotic therapy to be associated with the CPB group (Hosmer Lemeshow χ2, 7.11; P = .525), whereas the only variables associated with the CPB group in the comparison with the ESBL K. pneumoniae group were female sex and hospitalization abroad (Hosmer-Lemeshow χ2, 7.74; P = .459) (Table 3).
Table 3. Subgroup Analysis of Patients With Detection of CPB Versus Patients With Detection of Only ESBL E. coli (A) and Versus Patients With Detection of Only ESBL K. pneumoniae (B)

Note. CPB, carbapenemase-producing bacteria; ESBL-PE, extended-spectrum β-lactamase–producing Enterobacterales; OR, odds ratio; CI, confidence interval; ICU, intensive care unit. Bold indicates statistical significance.
a Home, other acute-care facility or nursing home.
b Within the previous 12 mo.
c Within 30 d prior to CPB detection.
d To be in place for at least 7 days prior to CPB detection.
e Refers to ESBL-PE and/or CPB in the CPB group and to only ESBL-PE in the ESBL-PE group.
f Within 3 mo prior to CPB detection.
Sensitivity analyses
Both sensitivity analyses (only considering patients a) colonized with carbapenemase-producing Enterobacterales and b) detected between January 2016 and December 2018), confirmed both hospitalization abroad and history of antibiotic therapy being associated with colonization with CPB compared to ESBL-PE (Supplementary Tables 4 and 6 online) and compared to ESBL E. coli (Supplementary Tables 5 and 7 online). Hospitalization abroad was inconsistently associated with colonization with CPB compared to ESBL K. pneumoniae. Both sensitivity analyses revealed that exposure to proton-pump inhibitors (PPIs) differed between these 2 groups (Supplementary Tables 5 and 7 online).
Discussion
In a setting of low endemicity with only sporadic cases of CPB, colonization with CPB could be related to travel abroad in 62% of all patients and any previous hospitalization in 84% of all patients. Moreover, 38% of patients were likely to have acquired colonization in Switzerland. Of concern, no exposure to healthcare settings or travel abroad was recorded for 12% of patients, pointing to potential community CPB transmission. Hospitalization abroad and recent antibiotic therapy were associated with increased risk of CPB in our setting of low CPB endemicity compared to patients with detection of ESBL-PE. The epidemiology of ESBL K. pneumoniae seems more comparable to the epidemiology of CPB rather than ESBL E. coli in terms of antibiotic exposure. This finding highlights the importance of selection pressure in addition to exposure in healthcare settings for successful colonization with both CPB and ESBL K. pneumonae compared with ESBL E. coli.
An analysis of our CPB cohort suggests 2 different epidemiologic characteristics. One comprises patients with travel history within the past 12 months, accounting for 2/3 of our study population. Furthermore, these patients were frequently in contact with a foreign healthcare environment, supporting probable introduction of CPB into Switzerland and hereby screening recommendations for repatriated patients or patients with known hospitalization abroad in the prior months. Reference Magiorakos, Burns and Rodriguez Bano17 Interestingly, recent local data documented stabilization in CPB rates in 2020–2021, which might reflect lower travel activities due to the COVID-19 pandemic. Reference Gasser18
The second epidemiologic subgroup comprised patients without travel history, who were more likely to have a history of CPB or ESBL-PE colonization and/or infection within the past year and were more likely to have been exposed to immunosuppressive therapy. This finding points to a population of patients with higher vulnerability and therefore possibly to a higher frequency of healthcare contacts. The size of this patient population is likely to increase in future decades due to medical progress and an aging population benefiting from advanced therapies. Thus, the need for constant evaluation of local CPB epidemiology on a national or even institutional level is underscored to adapt screening recommendations.
In most cases within both subgroups, CPB carriage was detected in clinical samples rather than admission or routine screening samples. Although recommendations for admission screenings exist in most Swiss hospitals, screening triggers and sampling sites vary widely among between Swiss healthcare institutions, as reported by Martischang et al. Reference Martischang, Buetti, Balmelli, Saam, Widmer and Harbarth19 Only 115 hospitals (78%) targeted CP Enterobacterales specifically, underscoring the need to improve CPB screening strategies.
In both CPB subgroups, OXA genotypes were the most prevalent followed by KPC, which is in line with recent national 1 as well as European data. Reference van Duin and Doi20,Reference Logan and Weinstein21 However, the CPB travel subgroup was associated with a significantly higher percentage of OXA genotypes, possibly associated with an also significantly higher amount of detection of CP A. baumannii in the CPB travel subgroup, highlighting the emergence of OXA-producing A. baumannii in Mediterranean countries, 22,Reference Djahmi, Dunyach-Remy, Pantel, Dekhil, Sotto and Lavigne23 which account for almost 65% of travel destinations in our cohort (Supplementary Table 8 online).
When comparing the CPB group to the ESBL-PE group, hospitalization abroad and previous antibiotic exposure were associated with carriage of CPB. Furthermore, these differences in epidemiology of the CPB cohort versus the ESBL-PE cohort derive mainly from comparisons to patients with detection of ESBL E. coli, as revealed by our subgroup analysis. Previous antibiotic exposure did not differ between patients colonized with CPB and ESBL K. pneumoniae. This finding highlights the importance of selection pressure in addition to exposure within healthcare settings for successful colonization compared to ESBL E. coli or continuing re-exposure within the community to ESBL E. coli. In contrast to ESBL E. coli, ESBL K. pneumoniae is more frequently related to nosocomial acquisition. Reference Scheuerman, Schechner and Carmeli24 Thus, infection prevention and control measures should be tailored to control transmission of ESBLs to the respective species. Reference Tschudin-Sutter, Lucet, Mutters, Tacconelli, Zahar and Harbarth25 Female sex was related to detection of CPB rather than ESBL K. pneumoniae in our cohort, and PPI use was associated with ESBL K. pneumoniae rather than CPB in both sensitivity analyses. Both associations remain unclear at this point, especially because PPI use has been associated with an increase in risk of acquisition of both CPB and ESBL producers. Reference Willems, Schut and Kaiser26
This study had several limitations. Due to the retrospective, single-center design, data might not be applicable to other settings and institutions especially in settings of high endemicity. The small sample size of the CPB group might have limited the detection of further predictors. Because assessment of travel history might be biased in favor of patients with very recent stay abroad, repatriation or patients questioned for infection of unknown origin, a lack of admission screening might have led to underdetection of CPB and underestimation of the proportion of travel history in CPB-positive patients. The differing timeframes of data acquisition of the CPB group (11 years) and the ESBL-PE group (3 years) may have precluded consideration of possible changes of epidemiology. To address this shortcoming, we performed sensitivity analyses considering only the same timeframes. Last, our study was designed to specifically study the epidemiology of β-lactamases (ESBLs and carbapenemases) conferring durable resistance rather than inducible resistance-mechanisms such as efflux-pump upregulation or porin loss. To address species-related differences between Enterobacterales and nonfermenters, additional sensitivity analyses were performed.
In conclusion, this study confirms low yet increasing rates of CPB carriage in Switzerland. Although CPB is still being mainly imported from areas of higher endemicity, local acquisition of CPB is emerging, especially in patients with close and/or frequent contact with healthcare services. Thus, frequent evaluation of CPB epidemiology on national and/or institutional levels is required to improve detection of patients at risk of CPB carriage.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/ice.2023.125
Acknowledgments
Financial support
This study was supported by the University Hospital Basel and the University of Basel.
Competing interests
All authors declare no competing interests related to this article.






