To the Editor—Prophylactic exchange of central venous catheters (CVC) for prevention of CVC-related bloodstream infections (CRBSI) in cancer patients is not generally recommended.Reference O’Grady, Alexander and Burns 1 , Reference Hentrich, Schalk and Schmidt-Hieber 2 The related studies were conducted in relatively small populations in general and especially in small populations of cancer patients; in addition, thrombocytopenia, which often occurs in cancer patients, was an exclusion criterion in these trials.Reference Cobb, High and Sawyer 3 , Reference Cook, Randolph and Kernerman 4 However, prophylactic CVC exchange is sometimes still clinical practice in hematology, even though the optimal time point is unclear. Therefore, we aimed to investigate this question in a larger cohort of patients with hematological malignancies.
For this purpose, pooled data from the prospective Study to Evaluate Central venous Catheter-related Infections in Hematology and Oncology (SECRECY) registry (German Clinical Trial Register No. DRKS00006551)Reference Schalk, Hanus, Färber, Fischer and Heidel 5 and the prospective Antimicrobial Catheter Securement Dressings for the Prevention of CVC-related Bloodstream Infections in Cancer Patients (COAT) study (ClinicalTrials.gov No. NCT01544686)Reference Biehl, Huth and Panse 6 from 11 centers in Germany were analyzed. SECRECY is an ongoing real-life registry of CRBSI in patients with hematological and oncological malignancies. COAT was a randomized multicenter trial comparing different CVC dressings in terms of CRBSI incidence in neutropenic patients.
In this study, we analyzed CRBSI due to short-term CVC (≥1 day in situ) inserted in the jugular or subclavian vein in patients with hematological malignancies. Only definitive CRBSI (dCRBSI) and the combination of definitive and probable CRBSI (dpCRBSI) according to the 2012 Infectious Diseases Working Party of the German Society for Hematology and Medical Oncology (AGIHO/DGHO) criteriaReference Hentrich, Schalk and Schmidt-Hieber 2 were considered from both data subsets. Using a receiver operating characteristic, CVC duration was used to determine a cutoff time point for CRBSI risk. An area under the curve (AUC) of <0.500 and 0.500–0.700 were considered of no and low predictive significance, respectively.Reference Šimundić 7
Altogether, 1,194 CVC patients (median age, 59 years; range, 18–86; 59.2% men) with 20,330 CVC days (median CVC duration, 17 days; range, 1–60) were analyzed. In total, 610 CVC patients (51.1%) were from the COAT study and 584 (48.9%) were from the SECRECY registry. Underlying diseases were acute leukemia in 568 of these patients (47.6%), multiple myeloma in 316 patients (26.5%), and lymphoma in 226 patients (18.9%). The insertion site was the jugular vein in 819 of these patients (68.6%) and the subclavian vein in 375 patients (31.4%). In 890 of these patients (74.5%), chlorhexidine-containing CVC dressings were used from the beginning of the CVC insertion.
In total, 55 dCRBSIs and 137 dpCRBSIs occurred. Definitive CRBSI originated in the jugular vein CVC in 26 of these 55 patients (47.3%); dpCRBSI originated in the jugular vein CVC in 87 of 137 dp CRBSI patients (63.5%). The epidemiological data are summarized in Table 1. The CVC duration was the same for CVC with dCRBSI and dpCRBSI (median, 16 vs 16 days; P=.62). No significant difference was detected between dCRBSI onset and dpCRBSI onset (median, 14 vs 13 days; P=.24). Comparing dCRBSI onset with dpCRBSI onset in jugular vein CVC, we also found no significant difference (median, 14 vs 13 days; P=.15). We also found no significant difference between dCRBSI onset and dpCRBSI onset in subclavian vein CVCs (median, 14 vs 13 days; P=.83) (Table 1). There was also no difference in dCRBSI onset between jugular vein and subclavian vein (median, 14 [range, 3–39] vs 14 days [range, 4–40]; P=.52) and for dpCRBSI (median, 13 [range, 2–39] vs 13 days [range, 4–40]; P=.66), respectively.
TABLE 1 Epidemiology and Characteristics of Central Venous Catheter (n=1,194) and Central Venous Catheter-Related Bloodstream Infection
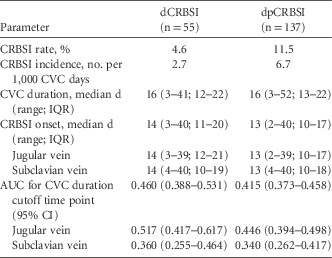
NOTE. CVC, central venous catheter; CRBSI, central venous catheter-related bloodstream infection; dCRBSI, definite CRBSI; dpCRBSI, combination of definite and probable CRBSI; IQR, interquartile range; AUC, area under the curve; 95% CI, 95% confidence interval.
For the CVC duration cutoff time point, an AUC of 0.460 for dCRBSI and an AUC of 0.415 for dpCRBSI were calculated. Considering only CVCs inserted in the jugular vein, an AUC of 0.517 for dCRBSI and an AUC of 0.446 for dpCRBSI were calculated. Furthermore, considering subclavian vein CVCs only, the AUCs for dCRBSI and dpCRBSI were 0.360 and 0.340, respectively (Table 1).
In conclusion, in this large cohort of patients with hematological malignancies and high risk for CRBSI, we could not determinate an optimal cutoff time point at which a prophylactic CVC exchange should be implemented in clinical care to prevent CRBSI, irrespective of the CVC insertion site (jugular vein or subclavian vein) or the strength of CRBSI definition (dCRBSI or dpCRBSI). The main reason for this finding is the very wide range of CRBSI onset. In all but 1 calculation, the AUC for CVC duration cutoff time point was <0.500; for dCRBSI and jugular vein CVC, the AUC was 0.517, but the lower bound of the 95% confidence interval was also <0.500. Therefore, CVC duration was of nonpredictive significance. Of course, the CRBSI risk increases with CVC duration.Reference Schalk, Hanus, Färber, Fischer and Heidel 5 , Reference Pepin, Thom and Sorkin 8 Therefore, decision making for preventive CVC removal or exchange is based on experience of the clinicians so far. A risk score at CVC insertion would be helpful to identify high-risk CVCs.
ACKNOWLEDGMENT
Financial support: No financial support was provided relevant to this article.
Potential conflicts of interest: L.M.B. has received lecture honoraria from Astellas and MSD travel grants from 3M and Gilead. M.J.G.T.V. has served at the speakers’ bureau of Pfizer, Merck, Gilead Sciences, Organobalance, Falk Foundation, and Astellas Pharma, has received research funding from 3M, DaVolterra, MSD/Merck, Astellas Pharma, Seres Therapeutics and Gilead Sciences, and is a consultant to Berlin Chemie and DaVolterra. All other authors report no conflicts of interest relevant to this article.



