Introduction
Urinary tract infections (UTIs) were the most common healthcare-associated infection (HAI) in 2002 accounting for 36% of all HAIs. Reference Klevens, Edwards and Richards1 However, the HAI landscape has changed substantially—a 2015 point-prevalence analysis found catheter-associated urinary tract infections (CAUTIs) to be the fifth most common HAI. Reference Magill, O’Leary and Janelle2 The decline in hospital-onset UTIs (HOUTIs) may be attributed in part to preventability of CAUTI Reference Umscheid, Mitchell, Doshi, Agarwal, Williams and Brennan3 and the availability and adoption of guidelines for mitigating those infections. Reference Gould, Umscheid, Agarwal, Kuntz and Pegues4
Changes to the definition of CAUTI in 2009 and 2015 also likely contributed to the decline in prevalence. The first definition modification removed asymptomatic bacteriuria, Reference Dudeck, Horan and Peterson5 and the second excluded both urine cultures that were positive for non-bacterial pathogens and those with colony counts below 100,000 colony-forming units per milliliter (CFU/mL). 6 The CAUTI definition updates appear primarily responsible for the decline in CAUTI rates, Reference Sopirala, Syed, Jandarov and Lewis7 while not seeming to impact positive urine cultures rates. Reference Press and Metlay8
The definition for CAUTI is stringent so it is likely that the majority of HOUTIs are non-CAUTI. 9 With pay-for-performance metrics focused on CAUTIs, these non-CAUTI HOUTIs have not been studied extensively. Prior research has shown a definitional change in CAUTI can increase the cases of reportable central line-associated bloodstream infection (CLABSI) events. Reference Fakih, Groves, Bufalino, Sturm and Hendrich10 Understanding how non-CAUTI HOUTIs are associated with secondary bloodstream infections may be important in light of the new, proposed HAI metric for hospital-onset bacteremia and fungemia (HOB) that is under development. Reference Yu, Ye and Edwards11
This analysis divides HOUTIs into two groups: 1) CAUTIs and 2) non-CAUTI HOUTIs and characterizes the clinical and economic burden of each group, examining their impact upon secondary HOB, total hospital costs, and length of stay (LOS).
Methods
Study design and population
This was a retrospective, observational study that utilized data from the Becton, Dickinson and Company (BD) Insights Research Database (BD, Franklin Lakes, New Jersey) from October 2015 to June 2019, which includes electronically captured laboratory, pharmacy, patient demographic, admission, discharge, and transfer data. Details of the data collection system have been previously described. Reference Yu, Jung and Ai12,Reference Yu, Ye and Edwards13
The facility-level aggregate burden of HOUTIs, and association between HOUTIs and secondary HOB, were estimated in adults aged 18 years or older with hospital LOS between 2 and 365 days without other HAIs (Cohort 1). Financial data, including actual cost of care for an admission, were available on a subset of hospitals so a second cohort was constructed. The attributable hospital cost and LOS of CAUTI and non-CAUTI HOUTIs were estimated in a nested sample (Cohort 2). Recognizing that patients who get CAUTI or non-CAUTI HOUTIs are different, several additional inclusion criteria were applied to improve comparability. The control group was comprised of patients meeting the following criteria: (1) LOS ≥2 days; (2) no antimicrobial therapy ≥72 hours following admission; (3) no diagnosis-related group (DRG) for infection; and (4) no other positive culture collected during the hospitalization period. Reference Brossette, Hacek and Gavin14,Reference Ridgway, Sun, Tabak, Johannes and Robicsek15 Patients with missing intensive care unit (ICU) status and those where the age distribution, major diagnostic category (MDC), DRG, or primary procedure were different from patients with CAUTI were excluded.
Exposure measures
Patients with HOUTI were defined using an algorithm which included: (1) presentation with a positive urine culture; (2) containing ≤2 non-commensal isolates (>10,000 colony-forming units/ml), on day 3 or later (ie, hospital-onset period); and (3) likely antimicrobial susceptibility testing (AST) predicated on the mechanism of specimen collection and identified pathogen consistent with the American Society of Microbiology’s (ASM) Clinical Microbiology Procedures Handbook. Reference Yarbrough and Potter16 In addition to a positive urine culture of a likely hospital-onset infection, all HOUTIs in this analysis were required to have a second qualification in order to maximize likelihood of clinical significance: a new antimicrobial, appropriate for managing the uropathogen, prescribed within 2 days of the collection date of the first positive urine culture. The time frame of the new antimicrobial use is consistent with the Centers for Disease Control and Prevention (CDC) Sepsis Surveillance Toolkit 17 and defined elsewhere. Reference Yu, Ye and Edwards11 CAUTI was defined using the National Healthcare Safety Network (NHSN) definition and was confirmed by hospital infection preventionists. 18 Non-CAUTI HOUTIs were defined as those HOUTIs not meeting the CAUTI definition (Table 1).
Table 1. Prevalence analysis of all cases of HOUTI (Cohort 1)

Outcome measures
Potential secondary HOB was defined as a non-contaminated, positive blood culture with a noncommensal HOB/sepsis pathogen, occurring 2 days before or 4 days after the positive urine specimen requiring antimicrobial susceptibility testing and presenting with the same pathogen. Reference Yu, Ye and Edwards11 All-cause 30-day readmission, mortality, LOS, and total cost of care to the organization were analyzed.
Other variables
Age, gender, insurance type, and hospital characteristics were assessed. The ALaRMS score, a severity of illness marker derived from laboratory values which calculates mortality risk during the same hospital admission, Reference Tabak, Sun, Nunez and Johannes19 was used to assess the clinical severity of hospitalized patients.
Statistical analysis
In Cohort 1, the prevalence of CAUTI and non-CAUTI HOUTI were estimated and the association with secondary HOB was evaluated. Patient and hospital characteristics were described overall and by CAUTI, non-CAUTI HOUTI, or control status using frequencies. The risk of secondary HOB, determined by examining the prevalence of HOB in patients with CAUTI and non-CAUTI HOUTI, was quantified. Analyses were stratified by ICU status to understand whether the location of the positive urine collection varied on the risk of HOB.
In Cohort 2, the attributable cost of CAUTI and non-CAUTI HOUTI compared to infection-free controls were estimated using generalized linear mixed models with hospital as random effect to account for within-cluster correlation of data, and adjusted for age, gender, insurance type, ALaRMS severity of illness score, hospital bed-size, teaching status, and urbanicity. For binary outcomes, a binomial regression was used. Poisson regression was used for LOS. Gamma regression was employed for total cost as it was skewed. Since ICU status Reference Halpern and Pastores20 and HOB Reference Yu, Jung and Ai12 are known to be associated with higher costs, analyses in Cohort 2 were a priori stratified by ICU status (the patient was never admitted to the ICU during their entire LOS—“never,” or the patient spent some portion of their stay in the ICU—“ever”), LOS (≤10 days or >10 days), and potential secondary HOB (yes or no).
All analyses were conducted using R software version 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) with RStudio (Boston, Massachusetts).
Ethical considerations
The study was approved as a limited retrospective data set for epidemiological analyses, exempted from consent by the New England Institutional Review Board/WCG and Human Subjects Research Committee (Wellesley, Massachusetts). It was conducted in compliance with Health Insurance Portability and Accountability Act requirements.
Results
Prevalence Cohort (Cohort 1)
In Cohort 1 of 549,433 hospitalizations (43 hospitals) (Supplemental Table 1), 5,193 patients (0.9%) met the criteria for CAUTI or non-CAUTI HOUTI. Of those 5,193 patients, 463 (8.9%) had CAUTI confirmed by infection preventionists at the facilities. A total of 434 (93.7%) of the CAUTI cases, and 3,177 (67.2%) of the non-CAUTI HOUTI cases, met the criteria for receipt of a new antimicrobial likely targeted at the uropathogen (Table 1; Figure 1A). An analysis by pathogen is presented in Supplemental Table 2.

Figure 1. Distribution of HOUTI and attendant risk of secondary HOB. A. Distribution of HOUTI (Prevalence Cohort—Cohort 1). B. Cases of Likely Secondary HOB in Patients with HOUTI (Prevalence Cohort—Cohort 1).
Association between CAUTI and non-CAUTI HOUTI with potential secondary HOB
The overall prevalence of likely secondary HOB was 3.7% in patients with CAUTI and non-CAUTI HOUTI. The risk of likely secondary HOB was higher in CAUTI compared to non-CAUTI HOUTI (7.8% vs 3.2%); however, the volume of HOB was threefold greater for non-CAUTI HOUTI compared to CAUTI (101 vs 34, Figure 1B). The location of urine specimen collection was more likely to be in the ICU for CAUTI with secondary HOB compared to non-CAUTI HOUTI with secondary HOB (55.9% vs 30.7%) (Table 1).
Attributable burden cohort (Cohort 2)
In Cohort 2 of 82,787 hospitalizations (40 hospitals) (Supplemental Table 1), 360 patients (0.4%) had CAUTI and 1,513 (1.8%) had non-CAUTI HOUTI. The mean age was 65.6 years (SD = 15) with the majority of patients being female (52.6%). Patients with CAUTI and non-CAUTI HOUTI were more likely to come from large academic hospitals in urban settings compared to control patients. The ALaRMS score for the same admission mortality risk was highest in CAUTI (M = 56.8, SD = 21.6) followed by non-CAUTI HOUTI (M = 56.4, SD = 19.8) then controls (M = 46.2, SD = 18.8) (Supplemental Table 3).
Attributable cost of CAUTI and non-CAUTI HOUTI
The attributable risk of increased LOS and total cost were higher in patients with CAUTI and non-CAUTI HOUTI compared to controls, any differential was less clear for readmission and mortality. The overall total adjusted hospital costs for CAUTI patients and their controls were $24,289 (95% CI: $19,861–$29,703) and $14,482 (95% CI: $12,488–$16.794), respectively, yielding an average incremental cost of $9,807 (P < .0001). For non-CAUTI HOUTIs and their controls, the overall total adjusted hospital costs were $21,363 (95% CI: $18,124–$25,180) and $14,489 (95% CI: $12,422–$16,899), respectively, yielding an average incremental cost of $6,874 (P < .0001) (Table 2).
Table 2. Overall findings from the attributable burden Cohort (Cohort 2)

Note. P value: *<.05; **<.01; ***<.0001. Models were adjusted for age, sex, ALaRMS score, and hospital-level variables (payer, staffed bed size, teaching status, and urbanicity).
Higher attributable cost of CAUTI and non-CAUTI HOUTI compared to controls were generally true in subgroup analyses. However, the magnitude of the association differed. Patients who stayed in the ICU, tended to have worse outcomes. Additionally, the burden of CAUTI and non-CAUTI HOUTI was smallest in LOS ≤10 days with no secondary HOB (Table 3) and much higher in subgroups with LOS >10 days and/or HOB (Supplemental Tables 4 and 5).
Table 3. Association between CAUTI and non-CAUTI HOUTI with outcomes in patients with LOS ≤ 10 d and no secondary HOB

Note. P value: ns not statistically significant; *<.05; **<.01; ***<.0001. Models were adjusted for age, sex, ALaRMS score, and hospital-level variables (payer, staffed bed size, teaching status, and urbanicity). d, day.
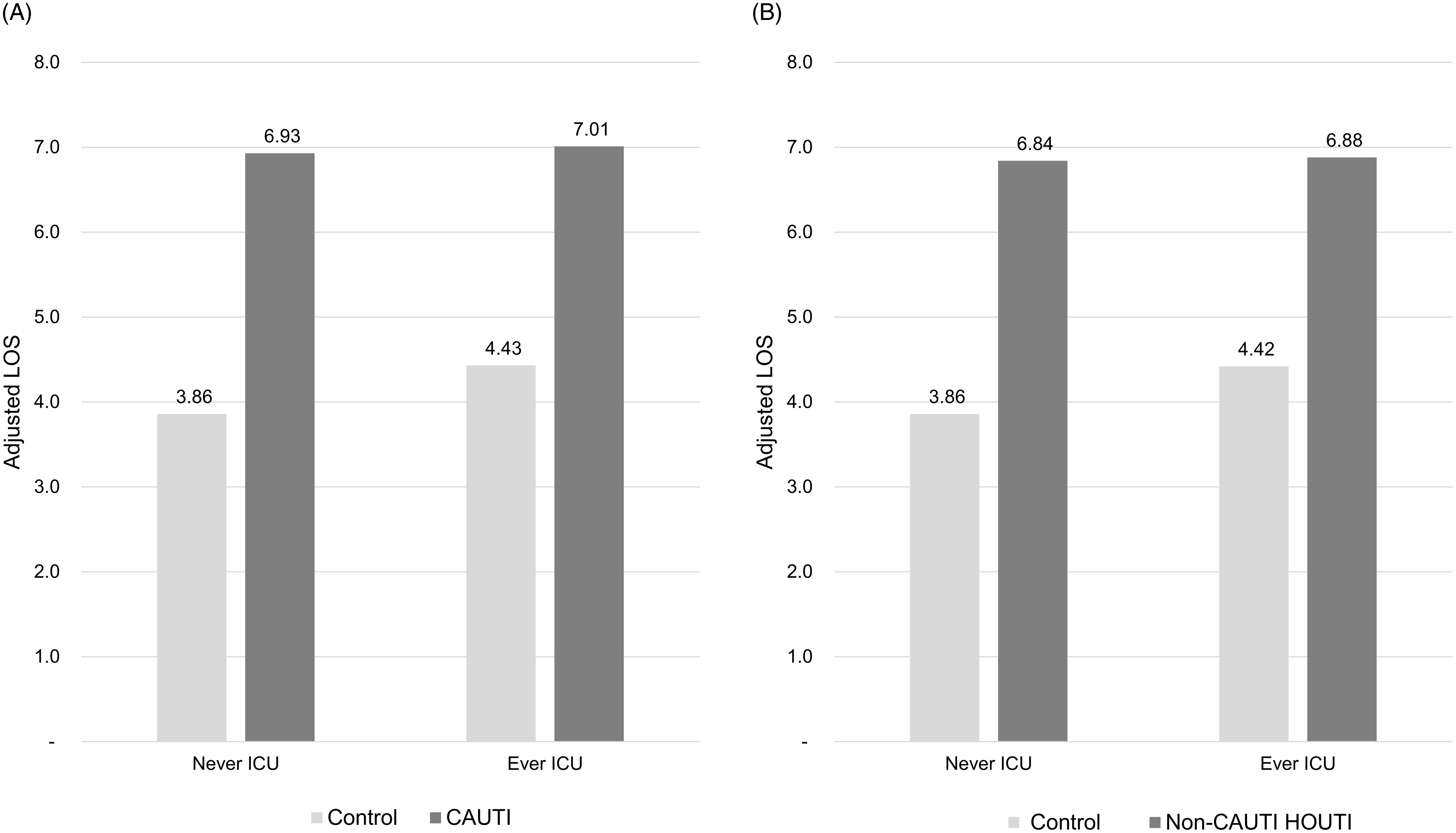
In the subgroup with LOS ≤10 days and no HOB, incremental adjusted total hospital cost was significantly higher for CAUTI patients: $5,439 (P < .01) with no ICU stay and $7,337 (P < .0001) with an ICU stay (Figure 2A). Similarly, non-CAUTI HOUTI patients had higher incremental adjusted total hospital costs: $6,101 (P < .0001) with no ICU stay and $5,966 (P < .0001) with an ICU stay (Figure 2B). Neither CAUTI nor non-CAUTI HOUTI had a significant impact on either 30-day readmission rates or mortality with the exception of CAUTI patients who had been in the ICU exhibiting a 2.14X greater risk of mortality than matched controls (P < .05, Table 3). Incremental adjusted LOS was significantly longer for CAUTI patients: 3.07 days (P < .0001) with no ICU stay and 2.58 days (P < .0001) with an ICU stay (Figure 3A). Similarly, non-CAUTI HOUTI patients had longer incremental adjusted LOS: 2.98 days (P < .0001) with no ICU stay and 2.46 days (P < .0001) with an ICU stay (Figure 3B).

Figure 2. Cost of CAUTI and non-CAUTI HOUTI. A. Adjusted total cost of CAUTI (Cohort 2—subjects with LOS ≤ 10 days, and no HOB). B. Adjusted total cost of non-CAUTI HOUTI (Cohort 2—subjects with LOS ≤ 10 days, and no HOB).

Figure 3. LOS Associated with CAUTI and non-CAUTI HOUTI. A. Adjusted LOS associated with CAUTI (Cohort 2—subjects with LOS ≤ 10 days, and no HOB). B. Adjusted LOS associated with non-CAUTI HOUTI (Cohort 2—subjects with LOS ≤ 10 days, and no HOB).
Discussion
In the general hospital population of adults, the proportion of non-CAUTI HOUTI was over 7-fold greater compared to CAUTI. The attributable risk of increased LOS and total hospital cost of both CAUTI and non-CAUTI HOUTI were significantly higher compared to controls. Though the risk of likely secondary HOB was higher in patients with CAUTI vs non-CAUTI HOUTI, the volume of HOB was three times greater in non-CAUTI HOUTI. This finding has implications for infection control programs as non-CLABSI HOB has been shown to sustain even higher incremental risk for longer LOS, higher hospital costs, and depending on ICU status, higher mortality and 30-day readmission risk. Reference Yu, Jung and Ai12
These findings highlight the importance of recognizing both CAUTI and non-CAUTI HOUTI and are complemented by a recent practice recommendation to establish a system for defining, analyzing, and reporting data on non-catheter-associated UTIs. Reference Patel, Advani and Kofman21 While CAUTI remains the more costly of the two infections, this analysis suggests that reducing not only CAUTI, but also non-CAUTI HOUTI, may result in improved patient outcomes and lower overall HOB rates. E. coli bloodstream infection rate is a UK National Health Service oversight metric 22 and research in the UK has found that E. coli bacteremia is most frequently caused by CAUTI. Reference Melzer, Wickramasinghe and Welch23 This is worthy of attention given that the Centers for Medicare & Medicaid Services is currently reviewing an all-cause HOB metric. Reference Stack, Dbeibo, Fadel, Kelley, Sadowski and Beeler24
As the definition for CAUTI has become more stringent, the prevalence of CAUTI has declined. Reference Sopirala, Syed, Jandarov and Lewis7,Reference Press and Metlay8,Reference Fakih, Groves, Bufalino, Sturm and Hendrich10 This study found that only 12.0% of all HOUTIs were identified as CAUTIs and thus the vast majority of HOUTIs did not meet currently reportable NHSN criteria. An analysis of cases of hospital-onset BSI, secondary to HOUTI, following introduction of the 2015 NHSN CAUTI definition changes, found that 41.9% of those HOB infections could not be classified as secondary to CAUTI because they were either fungal in nature or because the bacterial urine culture was <100,000 CFU/mL. Reference Greene, Ratz, Meddings, Fakih and Saint25 Our analysis builds upon these data, highlights the importance of both types of HOUTI and associated patient outcomes, and suggests a clinical opportunity for infection prevention efforts to focus on the more serious clinical outcomes of HOB via all-cause HOUTIs irrespective of indwelling urinary catheter use or limitations of pathogen levels and species.
Prior analyses of the cost of CAUTI have yielded low values. A 2010 Agency for Healthcare Research and Quality (AHRQ) estimate for the cost of CAUTI was $1,090 increasing more than 10-fold in seven years in a subsequent analysis. Reference Bysshe, Gao and Heaney-Huls26 The researchers noted that efforts to reduce the utilization of indwelling urinary catheters, and CAUTI definition changes, likely concentrated the remaining CAUTI cases to those patients with severe, more costly infections. Systematic reviews have previously estimated the cost of CAUTI to range from $1,768 to $10,197 (Medicare beneficiaries without and with ICU stays, 2016 USD) Reference Hollenbeak and Schilling27 to $13,793 (2015 USD). Reference Bysshe, Gao and Heaney-Huls26 This large range may be partially explained by the acuity associated with an extended LOS. Stratification by LOS and HOB during the hospitalization showed that the attributable cost of CAUTI was as low as $5,439 to $7,337 and was responsible for 2.58 to 3.07 additional LOS days in patients with LOS of ≤10 days and no matched HOB, depending on ICU status during the hospitalization.
This study is one of the first to quantify the attributable cost of non-CAUTI HOUTI. The attributable cost of non-CAUTI HOUTI was approximately $6,000 contributing 2.46 to 2.98 additional LOS days, depending on ICU status, in the least acute group of patients analyzed. This value is plausible and consistent with prior studies. Reference Tabak, Sung, Ye, Vankeepuram, Gupta and McCann28 It is within the lower end of the 95% CI range for CAUTI ($5,019 to $22,568, 2015 USD) set forth in the AHRQ report Reference Bysshe, Gao and Heaney-Huls26 and similar to the $6,424 (2011 USD) cost of a UTI admission determined by an analysis of the Nationwide Inpatient Sample, Reference Simmering, Tang, Cavanaugh, Polgreen and Polgreen29 both of which were published in 2017. We therefore augment these analyses with more recent outcomes and cost data.
This study had several limitations. The analyzed hospitals were limited to those where total cost of care per admission and NHSN HAI reporting were both readily available. The urine culture stewardship practices of the individual hospitals were unknown, for example, it is possible that cultures may have been ordered less frequently for patients with an indwelling catheter. The association between potential secondary HOB and HOUTI may have been underestimated due to the following: (1) application of a stringent definition of HOB which required it to occur on day 4 or later and within 48 hours of the first positive urine culture collection date, and (2) patients with admissions for infection were excluded potentially eliminating the highest acuity patients with HOUTI. The location of the urine specimen collection may not have been the true location of infection onset depending on patient movement between infection onset and when the specimen was obtained. In addition, with no access to notes in the medical record, the mechanism for collecting the urine specimen and/or the mechanism of urine management, including the use of indwelling urinary catheters, could not be ascertained (note that uropathogen growth ≥10,000 CFU/mL is considered significant in specimens obtained with an in-and-out catheter per ASM recommendations in the Clinical Microbiology Procedures Handbook Reference Yarbrough and Potter16 ), additional LOS could not be ascribed to the HOUTI with absolute certainty. Accounting for potential confounding was done at the design and analysis phase of the study. However, residual confounding may still be possible as with all observational studies. Finally, as the CDC definition of HOB is still evolving, the definition employed in this analysis is a signal of HOB burden and not the exact definition that may ultimately be used by the CDC and NHSN.
Despite these limitations, the study had several strengths. A stringent definition for non-CAUTI HOUTI required cases to have both: (1) an algorithmically derived indication of a likely hospital-onset UTI based on the positive urine culture, and (2) an order for complementary antimicrobials within 48 hours of that first positive culture (resulting in exclusion of 32.8% of HOUTIs to arrive at our cohort of 3,177 cases of non-CAUTI HOUTI). It is thus highly unlikely that asymptomatic bacteriuria was captured in any of the analyses. In addition, a large sample of US hospitals was utilized including sites with and without academic affiliations. Lastly, the database included both laboratory results and pharmacy orders providing a novel opportunity to evaluate non-CAUTI HOUTI and its association with HOB.
In conclusion, non-CAUTI HOUTI is a common and potentially preventable source for HOB that is associated with longer LOS and higher hospital costs compared to those in similar patients without an infection. While a higher percentage of CAUTI cases evolve into HOB, non-CAUTI HOUTI-related HOB occur more often. These data support the ongoing surveillance of not only CAUTI but also non-CAUTI HOUTI as the latter may also represent a vulnerable population that could benefit from surveillance and prevention efforts, particularly in the non-ICU setting.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/ice.2024.26.
Acknowledgements
None.
Author contributions
We thank Stephanie E. Tedford, PhD, of Pharmacologics, Inc., who, on the behalf of BD provided medical writing support.
Financial support
None reported.
Competing interests
All authors are employed by BD.