Approximately 1 in 25 patients contracts a healthcare-associated infection (HAI) each year in the United States; 75,000 die in the hospital as a result of their HAI.Reference Magill, Edwards and Bamberg 1 Pathogens that cause HAIs can spread in the hospital via healthcare providers (HCPs) and/or the environment.Reference Anderson, Chen and Weber 2 The complex interactions involved in pathogen transmission among patients, HCPs, and the environment are largely unknown.
Healthcare textiles, including curtains, sheets, and clothing, are frequently contaminated with epidemiologically important pathogens such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and Clostridium difficile. Reference Rutala, Gergen, Sickbert-Bennett, Williams and Weber 3 , Reference Mitchell, Dancer, Anderson and Dehn 4 Clothing of HCPs routinely becomes contaminated during clinical duties,Reference Burden, Cervantes, Weed, Keniston, Price and Albert 5 – Reference Loh, Ng and Holton 7 and it may serve as a source for transmission to patients or recontamination of the HCP or the environment.
Antimicrobial-impregnated textiles may decrease the acquisition and transmission of pathogens by HCP clothing. Preclinical data indicate that antimicrobial-impregnated textiles demonstrate activity against pathogens such as S. aureus, Candida albicans, Acinetobacter spp., vegetative Clostridium difficile, and Klebsiella pneumoniae.Reference Abdel-Mohsen, Abdel-Rahman, Hrdina, Imramovsky, Burgert and Aly 8 – Reference Mariscal, Lopez-Gigosos, Carnero-Varo and Fernandez-Crehuet 10 However, data to support the use of antimicrobial-impregnated textiles in clinical practice are limited.Reference Schweizer, Graham, Ohl, Heilmann, Boyken and Diekema 11 – Reference Boutin, Thom, Zhan and Johnson 15
We designed the Antimicrobial Scrub Contamination and Transmission (ASCOT) Trial (1) to determine whether antimicrobial-impregnated surgical scrubs decrease the burden of HCP clothing contamination compared to standard surgical scrubs following a 12-hour intensive care unit (ICU) shift and (2) to characterize the transmission dynamics of epidemiologically-important pathogens among the patient, the environment, and the HCP, a group we labeled the “transmission triangle.”
METHODS
Study Design and Participants
We performed this blinded, 3-arm, randomized controlled trial with crossover design in 2 ICUs at Duke University Hospital between June 15, 2015, and January 10, 2016. The study included 1 control arm and 2 intervention arms. Nurses wore standard cotton-polyester surgical scrubs in the control arm. In the Scrub 1 intervention arm, nurses wore surgical scrubs that contained a complex element compound with a silver alloy embedded in its fibers. In the Scrub 2 intervention arm, nurses wore surgical scrubs impregnated with an organosilane-based quaternary ammonium and a hydrophobic fluoroacrylate copolymer emulsion. Nurses participated in each arm during 3 consecutive 12-hour shifts in the ICU. Study personnel laundered all study scrubs 5 times and delivered them to nurse participants in plastic bags. Nurses donned the study scrubs at home and presented to work according to their routines.
In this study, we enrolled nurses from the medical ICU and surgical ICU at Duke University Hospital, a 936-bed tertiary-care hospital in Durham, North Carolina, where ICU nurses typically care for patients in 2 ICU rooms each shift. All nurses working in the medical ICU or the surgical ICU were eligible for enrollment. Data from enrolled nurses who failed to complete all 3 shifts were excluded. Patients known to be colonized or infected with any multidrug-resistant pathogen were routinely placed on contact precautions, during which all HCPs wore gowns and gloves upon entry into the room. These ICUs did not perform routine active surveillance during the study period.
The Duke University Health System Institutional Review Board approved the study. Nurses provided written informed consent. We received a waiver of informed consent to collect data and specimens from the patients receiving care from enrolled nurses. The study is registered on ClinicalTrials.gov (NCT 02645214).
Randomization and Blinding
After obtaining consent from the nurse subject, the study coordinator provided the nurse with 3 sets of scrubs. Nurses were blinded to the type of scrub. Brands and labels were removed from scrubs; all scrubs were “Duke blue” in color. The study coordinator provided written and verbal instructions on which scrubs to wear with each shift. Nurse participants were randomized to 1 of 6 sequences of scrubs (Supplementary Table 1). The nurses wore a different scrub type during each of the 3 12-hour shifts, so each nurse participated in each study arm in a 1:1:1 ratio. The study team was not blinded during specimen and data collection. However, microbiology personnel were blinded to study arm allocation during the analysis of specimens.
Procedures
The study coordinator arrived at the ICU prior to the beginning of each shift to obtain cultures from each nurse’s scrubs (sleeve, abdomen, and pocket) as well as specified “high touch” surfaces (bedrail, bed, and supply cart) in each of that nurse’s 2 assigned rooms (Supplementary Figure 1). The coordinator repeated the process of obtaining samples from each nurse’s scrubs and assigned rooms at the end of the nurse’s shift. The study coordinator also obtained cultures from each patient who received care from a participating nurse during that shift. Specimens were collected from the anterior nares, the perirectal area, and from the integument (i.e., wounds, drains, or axilla). Microbiological methods are provided in detail in the Supplementary Material.
Cultures from each nurse/patient/room grouping were analyzed for the presence of similar species. A potential transmission event or “acquisition” was defined as the new identification of a target pathogen on nurse clothing, the patient, or the environment. A confirmed transmission event or “transmission” was defined as an acquisition event for which a source from the transmission triangle could be identified and confirmed. Organisms involved in acquisition events were analyzed for transmission events using pulsed-field gel electrophoresis (PFGE; see Supplementary Material).Reference Sharma-Kuinkel, Rude and Fowler 16
Finally, each nurse completed a 4-question survey at the end of the shift to describe how the scrubs worn during the shift compared to their standard work scrubs. Ultimately, each nurse answered 3 surveys, corresponding with each arm in the study.
Outcomes
The primary outcome of our study was the change in total contamination on nurse scrubs, measured as the sum of colony-forming units (CFU) of bacteria identified on nurse scrubs from each clothing location. The following predetermined secondary outcomes were measured: (1) the presence or absence of individual target pathogens; (2) the number and proportion of acquisitions and transmission events; and (3) HCP perceptions of clothing.
Statistical Analysis
This prospective, blinded, randomized trial was designed to test the hypothesis that antimicrobial-impregnated scrubs would have a smaller increase in total contamination compared to control scrubs following a single 12-hour shift in the ICU. Data were summarized using standard statistical methods, as appropriate.
Power calculations were performed based on 2 primary assumptions. First, we assumed that the scrubs used in the control arm would have 2-log increase in total CFU from the beginning to the end of the shift (SD=2).Reference Bearman, Rosato and Elam 12 , Reference Burden, Keniston and Frank 13 Second, we assumed a within-nurse, between-shift correlation of 0.5 given the crossover design of the study. Based on these assumptions, we found that 40 subjects and 120 (40 subjects×3 shifts) repeated measures would provide 90% power to determine a mean 1-log decrease (SD=1) in HCP clothing contamination compared to the control. Power calculations were done with 2-sided significance level of 0.025 for each of the 2 primary comparisons.
We utilized a generalized estimating equation (GEE) linear regression model to compare the amount of contamination (log total CFU) between arms at the end of the shift. The model included type of scrubs, log total CFU in the beginning of shift, randomization sequence, patient characteristics (i.e., presence of a percutaneous drain, diarrhea, rectal tube present, or wound; the use of contact precautions; and mechanical ventilation), and total environmental contamination during the shift as covariates. An unstructured working correlation matrix was used. Adjusted mean difference estimates (compared to control) and 95% confidence intervals (CIs) were calculated for each intervention arm. The statistical significance for each of the 2 primary comparisons (each antimicrobial-impregnated scrub vs control) was corrected for multiple comparisons, and P<.025 was considered statistically significant. Responses to nurse questionnaires were compared using GEE logistic regression with type of scrubs as a covariate using unstructured working correlation matrix. We performed all statistical analyses using SAS version 9.4 software (SAS Institute, Cary, NC).
RESULTS
Randomized Controlled Trial Results
In total, 41 nurses were enrolled and randomized for study participation. We excluded 1 nurse because she failed to complete all 3 shifts due to illness. A total of 102 unique patients received care from 40 nurse subjects over 120 individual 12-hour ICU shifts and 167 patient encounters for an average of 1.4 patients per nurse per shift. Patients were generally similar across the 3 study arms (Table 1), though 4 of the patient characteristics were slightly less common in the Scrub 2 arm.
TABLE 1 Characteristics of 102 Unique Patients and 167 Patient Encounters During 120 ICU Shifts in the ASCOT Trial

NOTE. IQR, interquartile range; ICU, intensive care unit; MDR, multidrug-resistant; MRSA, methicillin-resistant Staphylococcus aureus; PEG, percutaneous endoscopic gastrostomy; VRE, vancomycin-resistant enterococci.
a Unless otherwise noted for an individual row. Numbers for each arm do not add up to the “overall” column because individual patients were often encountered in multiple arms.
b Scrubs contained a complex element compound with embedded silver-alloy.
c Scrubs impregnated with an organosilane-based quaternary ammonium and a hydrophobic fluoroacrylate copolymer emulsion.
In total, 2,919 cultures were obtained from the environment in patient rooms during the 120 shifts. Environmental contamination was generally similar in the rooms entered by nurses during each arm, though contamination was highest during the control arm (Table 2; supplemental Figure 2). Of the 3 environmental locations tested, bed rails had the highest amount of contamination, followed by beds and supply carts.
TABLE 2 Environmental Contamination Observed During 120 Shifts in the ASCOT Trial

NOTE. CFU, total number of colony-forming units defined as sum of colony-forming units from each cultured high touch surface; IQR, interquartile range.
a Scrubs contained a complex element compound with embedded silver-alloy.
b Scrubs impregnated with an organosilane-based quaternary ammonium and a hydrophobic fluoroacrylate copolymer emulsion.
A total of 2,185 cultures were obtained from HCP clothing during the 120 shifts. Our primary outcome, the increase in contamination of nurse clothing, was essentially unchanged across the 3 study arms (Table 3; Supplementary Figure 2). The median CFU increase was 61.5 (interquartile range [IQR], −3.0 to 191.0) in the control arm, 73.0 (IQR, −107.0 to 194.0) in the Scrub 1 arm, and 54.5 (IQR, −60.0 to 215.0) in the Scrub 2 arm. Nurses’ sleeves and clothing covering their abdomens showed the greatest increase in contamination during the shift.
TABLE 3 Change in Nurse Contamination During 120 Shifts in the ASCOT Trial: Overall Colony Forming Units (CFUs)
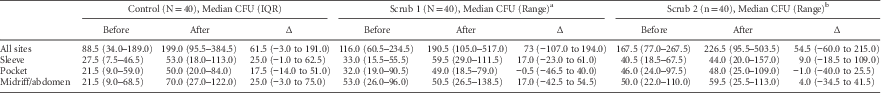
NOTE. IQR, interquartile range.
a Scrubs contained a complex element compound with embedded silver-alloy.
b Scrubs impregnated with an organosilane-based quaternary ammonium and a hydrophobic fluoroacrylate copolymer emulsion.
Scrub type was not associated with a change in HCP clothing contamination in multivariable linear regression that accounted for shift order, nurse crossover/clustering, pre-shift contamination, patient characteristics, and environmental contamination (overall P=.70). Mean difference estimates were 0.118 for the Scrub 1 arm (95% CI, −0.206 to 0.441; P=.48) and 0.009 for the Scrub 2 arm (95% CI, −0.323 to 0.342; P=.96).
Evaluation of Acquisition and Transmission Events
We identified acquisition events during 39 (33%) of the 120 shifts (Table 4), including 20 (17%) environmental acquisitions and 19 (16%) acquisitions on HCP clothing. Of all HCP clothing acquisitions, 3 (16%) occurred while caring for patients on contact precautions. The organisms most commonly involved in acquisition events were MSSA (n=11, 9%), Acinetobacter (n=10, 8%), and MRSA (n=8, 7%). Moreover, 23 of the acquisition events (59%) were confirmed transmission events from another member of the transmission triangle. The number of acquisition and transmission events were generally similar across study arms (Table 4).
TABLE 4 Bacterial Acquisition and Transmission EventsFootnote a During 120 Shifts in the ASCOT Trial

NOTE. Acq, acquisition; MSSA, methicillin-susceptible S. aureus, MRSA, methicillin-resistant S. aureus; Trans, transmission; VRE, vancomycin-resistant enterococci.
a An “acquisition event” was defined as contamination identified at the end of the shift that was not present at the beginning of the shift. A “transmission event” was defined as an acquisition event for which the source of contamination was identified and confirmed from another member of the transmission triangle. A nurse or the environment could have more than 1acquisition or transmission event during each shift.
b Scrubs contained a complex element compound with embedded silver-alloy.
c Scrubs impregnated with an organosilane-based quaternary ammonium and a hydrophobic fluoroacrylate copolymer emulsion.
Of the 19 HCP clothing acquisition events, 12 (63%) were confirmed transmission events: 7 from the patient, 3 from environmental contamination, and 2 from the patient and/or the environment (i.e., both the patient and environment were contaminated with target organism during the same shift) (Figure 1). Of these confirmed transmission events, 1 (8%) occurred while the patient was on contact precautions. Staphylococcus aureus was involved in the most HCP transmission events (n=6: 3 MRSA and 3 MSSA).

FIGURE 1 Description of 23 confirmed transmission events in the “transmission triangle” during the ASCOT study.
Of the 20 environmental acquisition events, 11 (55%) were confirmed transmission events. All 11 were transmitted from the patient. We did not identify any MDROs on HCP clothing at the beginning of a shift. Clothing from 3 HCP (7.5%) were contaminated with MSSA. No transmissions from HCP-to-patient or HCP-to-environment were observed. Finally, nurse perceptions of scrub types are provided in the Supplementary Material.
DISCUSSION
Transmission of microorganisms involves 3 elements: a source, a susceptible host with a portal of entry receptive to the agent, and a mode of transmission.Reference Siegel, Rhinehart, Jackson and Chiarello 17 In the healthcare setting, these elements culminate in pathogen movement between the patient, healthcare providers (HCP), and the environment, known as the “transmission triangle.” When transmission in this triangle leads to patient acquisition, healthcare-associated infections (HAIs) can follow.Reference Magill, Edwards and Bamberg 1 Our randomized controlled trial focused on the use of antimicrobial-impregnated scrubs as a strategy to decrease pathogen movement in the transmission triangle. Our results demonstrated that bacterial contamination of HCP clothing was not decreased when HCPs wore the antimicrobial-impregnated scrubs used in this study compared to standard (control) scrubs. We did, however, confirm that HCP clothing frequently became contaminated during routine clinical care, and pathogens were frequently acquired from the patient care environment.
Strategies to decrease pathogen movement in the transmission triangle can be generally categorized by the points of the triangle. Interventions can focus on the patient (eg, decolonization or source control), the environment (eg, enhanced disinfection, antimicrobial surfaces or textiles), or the healthcare provider (eg, hand hygiene, gowns and gloves, antimicrobial-impregnated clothing). Growing evidence suggests that contaminated healthcare textiles may be a source for transmission of epidemiologically important pathogens and HAIs.Reference Mitchell, Dancer, Anderson and Dehn 4 Preclinical data suggested that antimicrobial-impregnated textiles are efficacious at reducing bacterial burden,Reference Abdel-Mohsen, Abdel-Rahman, Hrdina, Imramovsky, Burgert and Aly 8 – Reference Mariscal, Lopez-Gigosos, Carnero-Varo and Fernandez-Crehuet 10 but data to support the use of antimicrobial-impregnated scrubs in clinical practice are limited. To date, 4 other trials have examined antimicrobial-impregnated scrubs, with conflicting results.Reference Bearman, Rosato and Elam 12 – Reference Boutin, Thom, Zhan and Johnson 15 Bearman et alReference Bearman, Rosato and Elam 12 performed a blinded, crossover, randomized controlled trial comparing antimicrobial-impregnated surgical scrubs to standard surgical scrubs. Use of antimicrobial-impregnated scrubs led to a>4 log10 reduction in the burden of MRSA on the HCP (P<.01), but no significant differences were detected in the amount of VRE or gram-negative bacilli contamination. In contrast, 3 previously published prospective randomized trials failed to show significant decreases in bacterial contamination in nurses or ambulance personnel.Reference Burden, Keniston and Frank 13 – Reference Boutin, Thom, Zhan and Johnson 15
Our methods helped provide a more complete analysis of this technology, as we measured and adjusted for environmental contamination and included important organisms such as MSSA and vancomycin-susceptible Enterococcus in our analyses. In light of these methodological advantages and the weight of the studies summarized above, we conclude that antimicrobial-impregnated scrubs are not efficacious at decreasing the bacterial burden on HCP during a single shift. We hypothesize that the lack of efficacy in this setting is related to the low-level disinfectant capabilities of the textiles coupled with repeated inoculation events and a short time frame (eg, a shift of 8 to 12 hours) in which to have an effect. Antimicrobial-impregnated textiles may be more effective in settings with potentially prolonged exposure and activity, including curtains and linens.Reference Schweizer, Graham, Ohl, Heilmann, Boyken and Diekema 11 , Reference Sifri, Burke and Enfield 18
Our study confirmed that HCP clothing regularly becomes contaminated with important pathogensReference Treakle, Thom, Furuno, Strauss, Harris and Perencevich 6 , Reference Rock, Thom, Masnick, Johnson, Harris and Morgan 19 , Reference Snyder, Thom and Furuno 20 and, as a result, can act as a source for transmission. For example, in a cohort study of 57 nurses, approximately half of the nurses’ surgical scrubs became contaminated with VRE, MRSA, and/or C. difficile by the end of a standard shift.Reference Perry, Marshall and Jones 21 Similarly, HCP clothing contaminated with S. aureus, Acinetobacter spp., and/or enterococci is associated with increased risk of contaminated HCP hands.Reference Munoz-Price, Arheart and Mills 22 Indeed, HCP clothing can become contaminated by interacting with either patients or the environment.Reference Morgan, Rogawski and Thom 23
Patients regularly contaminate their environment,Reference Thom, Johnson, Lee and Harris 24 , Reference Rutala, Gergen and Weber 25 which may be the biggest predictor of HCP contamination. Morgan et alReference Morgan, Rogawski and Thom 23 analyzed microbiological data from 585 HCP–patient interactions and concluded that positive environmental cultures led to a 4-fold increase in the risk of HCP contamination. In our study, the patient was the source of contamination for all organisms encountered in the environment. Nurses acquired important pathogens during 19% of shifts; we confirmed the source of transmission during 10% of shifts. Importantly, the environment served as the source of nurse contamination in 25% to 42% of transmission events in our study.
Our study has limitations. First, cultures obtained from clothing and the environment represented random sampling from these sites. While the use of replicate organism detection and counting (RODAC) plates has been validated for use on textiles,Reference Rutala, Gergen, Sickbert-Bennett, Williams and Weber 3 the surface area sampled using this technique may have failed to demonstrate the extent of contamination. Given the randomized nature of our study, we doubt this impacted our comparisons. Second, we obtained cultures from 3 patient locations, and we may have missed patient colonization with target pathogens in other locations. As a result, we summarized both acquisitions and confirmed transmissions in our study. Third, we did not account for pathogen-specific colonization pressure within participating ICUs in our models. However, this potential confounder would have had to shift dramatically within a short period (i.e., 24 hours) to impact our results. Finally, nurse behavior may have varied between study arms based on personal or patient-related concerns. We attempted to mitigate this risk using a crossover design, but we did not observe nurses during their shifts. Randomization led to equivalent patient numbers in each study arm.
Contamination of HCPs is an important component of pathogen transmission in healthcare settings. Results from our randomized controlled trial demonstrated that antimicrobial-impregnated scrubs were not efficacious at reducing nurse contamination and, thus, are not a useful strategy for stopping the movement of pathogens in the transmission triangle. We propose that future studies of antimicrobial-impregnated textiles focus on textiles that have frequent and long-term contact with patients, such as bed linens and gowns. Finally, our study demonstrated that the environment is an important source for HCP clothing contamination. We conclude that until additional data are available, the best strategies to reduce the risk of HCP clothing contamination remain diligent hand hygiene following all patient room entries and exits and, when appropriate, use of gowns and gloves, even if no direct patient care is performed.
ACKNOWLEDGMENTS
Financial support. This work was supported by grants from the Centers for Disease Control and Prevention (CDC) Prevention Epicenters Program (grant no. U54CK000164 to D.J.S.); the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (grant no. UL1TR001111 to V.G.F.); and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grant no. R01AI100560, R01AI063517, R21AI114508, and R01AI072219 to R.A.B.). Work performed during this study was also supported in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs (grant no. 1I01BX001974 to R.A.B.), by the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development, and by the Geriatric Research Education and Clinical Center VISN 10 to R.A.B. The views expressed in this article are those of the authors and do not necessarily represent the views of the CDC or NIH.
Potential conflicts of interests: Dr Rutala and Dr Weber report receiving consulting fees from Clorox. All other authors report no conflicts of interest relevant to this article.
SUPPLEMENTARY MATERIAL
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2017.181.







