Local transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus in Singapore has been reported. Reference Young, Ong and Kalimuddin1 As the pandemic spreads globally, increased utilization and shortages of personal protective equipment (PPE) are expected. Although extended PPE use would mitigate utilization rate, its safety is unknown. At the National Centre for Infectious Diseases, recommendations for healthcare workers (HCWs) in contact with known or suspected patients are in concordance with the US Centers for Disease Control and Prevention, which recommends gloves, gown, respiratory protection (eg, disposable N95 respirator), and eye protection (eg, goggles or disposable face shield), without the use of shoe covers. 2
An initial pilot study showed no contamination of N95 and disposable face visors after patient care, although in 1 instance, SARS-CoV-2 nucleic acid was detected on the front surface of an HCW’s shoe. Reference Ong, Tan and Chia3 To evaluate the safety of extended PPE use, we conducted a 1-day PPE sampling study on HCWs caring for confirmed COVID-19 patients to ascertain the per contact episode risk of PPE contamination with SARS-CoV-2.
Methods
The PPE samples were collected by 5 trained personnel using a standardized technique with Puritan EnviroMax Plus premoistened sterile swabs (Puritan Medical Products, Guilford, ME) from the entire front of goggles, the front surface of N95 respirator, and the front surfaces of shoes of 30 HCWs (Table 1) exiting patient rooms. Gloves and gowns were not swabbed because these are disposed after each use. Data on HCW category and details of activity in the room were recorded. Patients with positive SARS-CoV-2 PCR assays within the prior 48 hours were selected, and clinical data (ie, day of illness, presence of symptoms, and cycle threshold [Ct] value of clinical PCR) were obtained from the medical record. Environmental samples were tested using specific real-time RT-PCR methods targeting the SARS-CoV-2 RNA-dependent RNA polymerase (RdRP) and E genes. Reference Corman, Landt and Kaiser4
Table 1. Characteristics of PPE Samples Collected and Relevant Patient Clinical Data
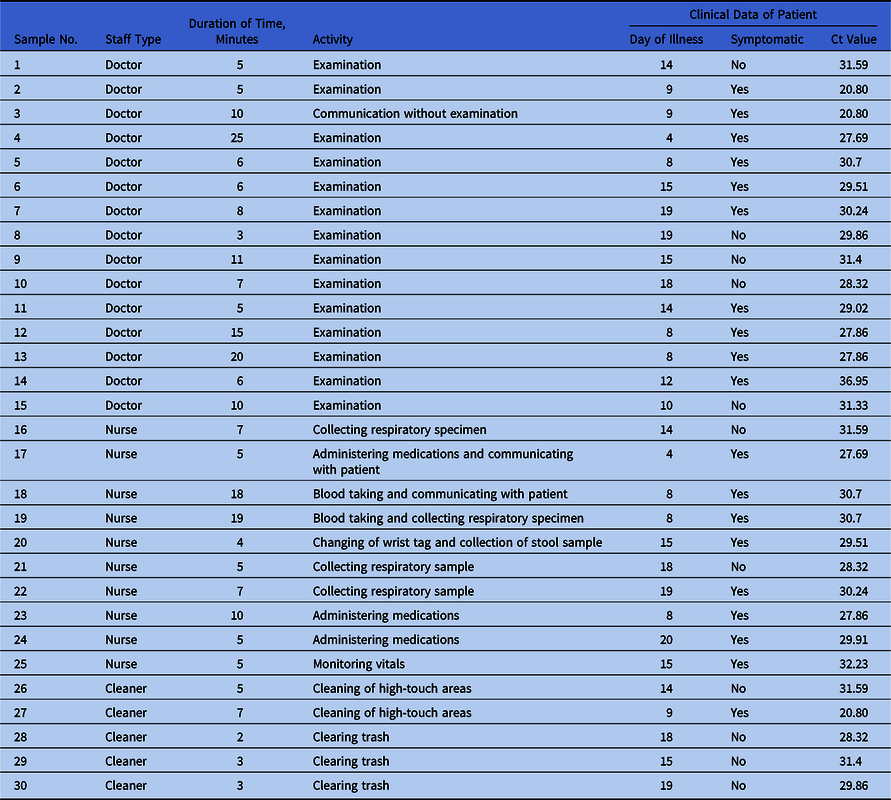
Note. Ct, cycle threshold. Cycle threshold refers to the number of cycles required for the fluorescent signal to cross the threshold in RT-PCR; a lower cycle threshold value indicates a higher viral load.
Results
In total, 15 patients (7 women and 8 men) were selected. Patient characteristics varied by day of illness (median, day 14; interquartile range [IQR], 8.25–17.25), presence of symptoms (63% symptomatic), and clinical PCR Ct value (median, 30.08; IQR 28.85–30.86). No patient required ventilatory support and no aerosol-generating procedures were carried out prior to or during sampling. All 90 samples from 30 HCWs (doctors, nurses, and cleaners) were negative (Table 1). The median time spent in the patient’s room overall was 6 minutes (IQR, 5–10): 8 minutes for doctors, 7 minutes for nurses, and 3 minutes for cleaning staff. Activities ranged from casual contact (eg, administering medications or cleaning) to closer contact (eg, physical examination or collection of respiratory samples).
Discussion
Our study had several limitations. One limitation of our study was the use of surface swabs for sampling the surface of N95 masks rather than processing masks in extraction buffers with detergents, which is a method that has been used for isolation of influenza from N95 respirators. Reference Blachere, Lindsley and McMillen5 Surface swabbing may be insufficient for the detection of entrapped viral particles. Second, all patients were in airborne infection isolation rooms with 12 air exchanges per hour, and these results may not be generalizable to other room configurations. Third, we did not assess the concomitant level of viral contamination of the environment in this study to correlate with the level of PPE contamination.
Previous laboratory studies have demonstrated that viruses, such as SARS-CoV and human coronavirus 229E, can remain viable on PPE items, including latex gloves and disposable gowns, Reference Lai, Cheng and Lim6–Reference Casanova, Rutala, Weber and Sobsey8 but these studies were not performed in clinical settings. Despite the potential for extensive environmental contamination by SARS-CoV-2, we did not find similar contamination of PPE after patient contact. These results provide assurance that extended use of N95 and goggles with strict adherence to environmental and hand hygiene while managing SARS-CoV-2 patients could be a safe option.
Acknowledgments
We thank Ding Ying and other members of the Infectious Diseases Research and Training Office for assistance with sample processing as well as all members of the National Centre for Infectious Diseases COVID-19 Outbreak Research Team: Poh Lian Lim, David Chien Lye, Cheng Chuan Lee, Li Min Ling, Lawrence Lee, Tau Hong Lee, Chen Seong Wong, Sapna Sadarangani, Ray Lin, Deborah HL Ng, Mucheli Sadasiv, Chiaw Yee Choy, Glorijoy Tan, Frederico Dimatatac, Isais Florante Santos, Go Chi Jong, Yu Kit Chan, Jun Yang Tay, and Pei Hua Lee. We also thank the DSO environmental detection team and Clinical diagnostics team for BSL3 sample processing and analysis; and logistics and repository team for transport of biohazard material, inventory, and safekeeping of received items.
Financial support
Funding for this study was provided by DSO National Laboratories. Ng Ot is supported by an NMRC Clinician Scientist Award (grant no. MOH-000276). Dr Kalisvar Marimuthu is supported by NMRC CS-IRG (grant no. CIRG18Nov-0034).
Conflicts of interest
All authors report no conflicts of interest relevant to this article.



