Introduction
Irritable bowel syndrome (IBS), with a worldwide prevalence of 4 per cent (female-to-male ratio of 2:1), is an extensively researched disorder of gut–brain interaction (DGBI), formerly known as functional gastrointestinal (GI) disorders (Sperber et al., Reference Sperber, Bangdiwala, Drossman, Ghoshal, Simren, Tack, Whitehead, Dumitrascu, Fang, Fukudo, Kellow, Okeke, Quigley, Schmulson, Whorwell, Archampong, Adibi, Andresen, Benninga, Bonaz, Bor, Fernandez, Choi, Corazziari, Francisconi, Hani, Lazebnik, Lee, Mulak, Rahman, Santos, Setshedi, Syam, Vanner, Wong, Lopez-Colombo, Costa, Dickman, Kanazawa, Keshteli, Khatun, Maleki, Poitras, Pratap, Stefanyuk, Thomson, Zeevenhooven and Palsson2021). The diagnosis is based on the clinical history and symptoms. According to the Rome IV criteria, these patients are defined by the presence of chronic or recurrent abdominal pain associated with defecation and/or altered bowel habits, in the absence of positive findings on the limited number of tests recommended to exclude organic diseases. Based on bowel habits, patients are categorised into predominant constipation (IBS-C), predominant diarrhoea (IBS-D), mixed bowel habits (IBS-M) or IBS unclassified (IBS-U) (Lacy et al., Reference Lacy, Mearin, Chang, Chey, Lembo, Simren and Spiller2016). Patients also often report concomitant symptoms and comorbidities such as bloating, abdominal distension, overlapping upper GI symptoms and extra-intestinal or psychological conditions (Enck et al., Reference Enck, Aziz, Barbara, Farmer, Fukudo, Mayer, Niesler, Quigley, Rajilić-Stojanović, Schemann, Schwille-Kiuntke, Simren, Zipfel and Spiller2016; Lacy et al., Reference Lacy, Mearin, Chang, Chey, Lembo, Simren and Spiller2016). IBS is not life-threatening but it greatly impacts the quality of life of the patients and via high health care consumption and reduced work productivity, also the society (Canavan et al., Reference Canavan, West and Card2014).
An intricate pathophysiology with absence of organic disease or well-defined biological markers is characteristic of this disorder with unknown aetiology (Enck et al., Reference Enck, Aziz, Barbara, Farmer, Fukudo, Mayer, Niesler, Quigley, Rajilić-Stojanović, Schemann, Schwille-Kiuntke, Simren, Zipfel and Spiller2016). Still, the gut-brain axis and its bidirectional interaction seem to be a cornerstone for symptom generation, hence the new term for these disorders, DGBI (Drossman, Reference Drossman2016). In addition, the generation of the hallmark IBS symptoms may be influenced by other factors, such as visceral hypersensitivity (Posserud et al., Reference Posserud, Stotzer, Björnsson, Abrahamsson and Simrén2007), altered GI motility (Simrén et al., Reference Simrén, Castedal, Svedlund, Abrahamsson and Bjõrnsson2000), increased intestinal permeability (Piche et al., Reference Piche, Barbara, Aubert, Varannes, Dainese, Nano, Cremon, Stanghellini, De Giorgio, Galmiche and Neunlist2009) and low-grade mucosal inflammation (Spiller, Reference Spiller2004), along with alterations in the gut microenvironment, including gut microbiota (Öhman et al., Reference Öhman, Törnblom and Simrén2015).
Gut microbiota and IBS
The link between the gut microbiota and IBS is supported by multiple studies and clinical observations. A proportion of patients with IBS develops symptoms following a resolved bacterial infection (post-infection IBS) (Barbara et al., Reference Barbara, Grover, Bercik, Corsetti, Ghoshal, Ohman and Rajilić-Stojanović2019), presumably linked to alterations of gut microbiota composition (Jalanka-Tuovinen et al., Reference Jalanka-Tuovinen, Salojärvi, Salonen, Immonen, Garsed, Kelly, Zaitoun, Palva, Spiller and de Vos2014). Further, the use of systemic antibiotics for non-GI conditions may also have a negative impact on gut microbiota and increase the risk of various DGBI (Paula et al., Reference Paula, Grover, Halder, Locke, Schleck, Zinsmeister and Talley2015). On the contrary, targeting gut microbiota using non-absorbable antibiotics or probiotics holds promise as a treatment strategy in IBS (Ford et al., Reference Ford, Harris, Lacy, Quigley and Moayyedi2018).
Even though there are inconsistent findings in the literature, a subset of IBS patients seems to display an altered gut microbiota composition compared with healthy individuals (Liu et al., Reference Liu, Wu, Chen, Chen, Shen and Liu2017; Pittayanon et al., Reference Pittayanon, Lau, Yuan, Leontiadis, Tse, Surette and Moayyedi2019), which can be associated with clinical and psychological parameters (Jeffery et al., Reference Jeffery, O’Toole, Öhman, Claesson, Deane, Quigley and Simrén2012), IBS severity or low microbial richness (Tap et al., Reference Tap, Derrien, Törnblom, Brazeilles, Cools-Portier, Doré, Störsrud, Le Nevé, Öhman and Simrén2017). Interestingly, specific bacteria have also been associated with IBS (eg. decrease of Bifidobacterium and Faecalibacterium and increase of the genus Bacteroides as compared to healthy subjects), as reviewed in Pittayanon et al. (Reference Pittayanon, Lau, Yuan, Leontiadis, Tse, Surette and Moayyedi2019). Pathogenic bacteria, such as Brachyspira, could potentially also play a role in the pathogenesis (Jabbar et al., Reference Jabbar, Dolan, Eklund, Wising, Ermund, Johansson, Törnblom, Simren and Hansson2021). Despite multiple studies indicating involvement of altered microbiota in IBS pathogenesis, the challenge lies in attributing disease causality to a gut microbiota profile or specific bacterial taxa. It is worth noting that gut microbiota in IBS seems unstable over time compared to healthy subjects (Mättö et al., Reference Mättö, Maunuksela, Kajander, Palva, Korpela, Kassinen and Saarela2005) and under the influence of exogenous factors (eg. diet and antibiotics) (Bhattarai et al., Reference Bhattarai, Muniz Pedrogo and Kashyap2017) as well as bowel habits that shift the intestinal microenvironment (Durbán et al., Reference Durbán, Abellán, Jiménez-Hernández, Artacho, Garrigues, Ortiz, Ponce, Latorre and Moya2013), making it unclear whether altered microbiota is a cause, consequence, or both, of IBS. Altogether, these findings contribute to the understanding of IBS and potentially facilitate the characterisation of patients in regards to prognosis and response to treatments in the foreseeable future (Jeffery et al., Reference Jeffery, O’Toole, Öhman, Claesson, Deane, Quigley and Simrén2012).
The link between metabolites and IBS
Metabolites ensure the host-microbiota crosstalk and are involved in various biological functions in the gut, including providing energy for epithelial cells, maintenance of intestinal barrier function and nutrient absorption (Nicholson et al., Reference Nicholson, Holmes, Kinross, Burcelin, Gibson, Jia and Pettersson2012). Earlier studies have indicated that some IBS patients have altered levels of specific metabolite classes, such as short-chain fatty acids (SCFAs), bile acids and amino acids (Duboc et al., Reference Duboc, Rainteau, Rajca, Humbert, Farabos, Maubert, Grondin, Jouet, Bouhassira, Seksik, Sokol, Coffin and Sabaté2012; Tana et al., Reference Tana, Umesaki, Imaoka, Handa, Kanazawa and Fukudo2010; Zhang et al., Reference Zhang, Zhang, Qin, Li, Wei, Li and Yao2019). More recent studies making use of untargeted metabolomics analyses have also revealed alterations in the metabolite profiles in serum (Xu et al., Reference Xu, Jia, Zhang, Wang, Zhu, Wang, Liu, Li, Zhang, Wang, Zhang, Sun, Wang, Zhu and Duan2020), urine (Jeffery et al., Reference Jeffery, Das, O’Herlihy, Coughlan, Cisek, Moore, Bradley, Carty, Pradhan, Dwibedi, Shanahan and O’Toole2020; Liu et al., Reference Liu, Gu, Li, Li, Li, Cui and Zuo2020), and faeces (Ahluwalia et al., Reference Ahluwalia, Iribarren, Magnusson, Sundin, Clevers, Savolainen, Ross, Törnblom, Simrén and Öhman2021; Jeffery et al., Reference Jeffery, Das, O’Herlihy, Coughlan, Cisek, Moore, Bradley, Carty, Pradhan, Dwibedi, Shanahan and O’Toole2020; Lee et al., Reference Lee, Kim, Chun, Chun, Shin, Choi and Choi2020; Ponnusamy et al., Reference Ponnusamy, Choi, Kim, Lee and Lee2011; Zhu et al., Reference Zhu, Liu, Li, Zhang, Zhang, Chen, Zhao, Chen, Gu, Min and Zhang2019) of IBS patients. While there is no clear consensus on these changes being the cause or effect of IBS, the metabolome alterations have been found to be associated with gut microbiota composition (Jeffery et al., Reference Jeffery, Das, O’Herlihy, Coughlan, Cisek, Moore, Bradley, Carty, Pradhan, Dwibedi, Shanahan and O’Toole2020; Lee et al., Reference Lee, Kim, Chun, Chun, Shin, Choi and Choi2020; Xu et al., Reference Xu, Jia, Zhang, Wang, Zhu, Wang, Liu, Li, Zhang, Wang, Zhang, Sun, Wang, Zhu and Duan2020; Zhu et al., Reference Zhu, Liu, Li, Zhang, Zhang, Chen, Zhao, Chen, Gu, Min and Zhang2019), IBS symptoms (Liu et al., Reference Liu, Gu, Li, Li, Li, Cui and Zuo2020; Xu et al., Reference Xu, Jia, Zhang, Wang, Zhu, Wang, Liu, Li, Zhang, Wang, Zhang, Sun, Wang, Zhu and Duan2020; Zhu et al., Reference Zhu, Liu, Li, Zhang, Zhang, Chen, Zhao, Chen, Gu, Min and Zhang2019) as well as psychological well-being (Liu et al., Reference Liu, Gu, Li, Li, Li, Cui and Zuo2020). Although the metabolome profile alone fails to discriminate between IBS subtypes (Jeffery et al., Reference Jeffery, Das, O’Herlihy, Coughlan, Cisek, Moore, Bradley, Carty, Pradhan, Dwibedi, Shanahan and O’Toole2020), combined with faecal microbiota pattern a separation between IBS-C and IBS-D can be observed (Ahluwalia et al., Reference Ahluwalia, Iribarren, Magnusson, Sundin, Clevers, Savolainen, Ross, Törnblom, Simrén and Öhman2021). Metabolite alterations during IBS flares further support the link between microbial metabolism and IBS severity, as shown in a recent longitudinal study (Mars et al., Reference Mars, Yang, Ward, Houtti, Priya, Lekatz, Tang, Sun, Kalari, Korem, Bhattarai, Zheng, Bar, Frost, Johnson, van Treuren, Han, Ordog, Grover, Sonnenburg, D’Amato, Camilleri, Elinav, Segal, Blekhman, Farrugia, Swann, Knights and Kashyap2020).
In this narrative review, we aim to provide an overview of the current strategies targeting the gut microenvironment that are proposed as treatment options for DGBI (Figure 1). Particularly we focus on studies assessing the effects on gut microenvironment, including microbiota and metabolites, and its interaction with clinical symptoms in patients with IBS.
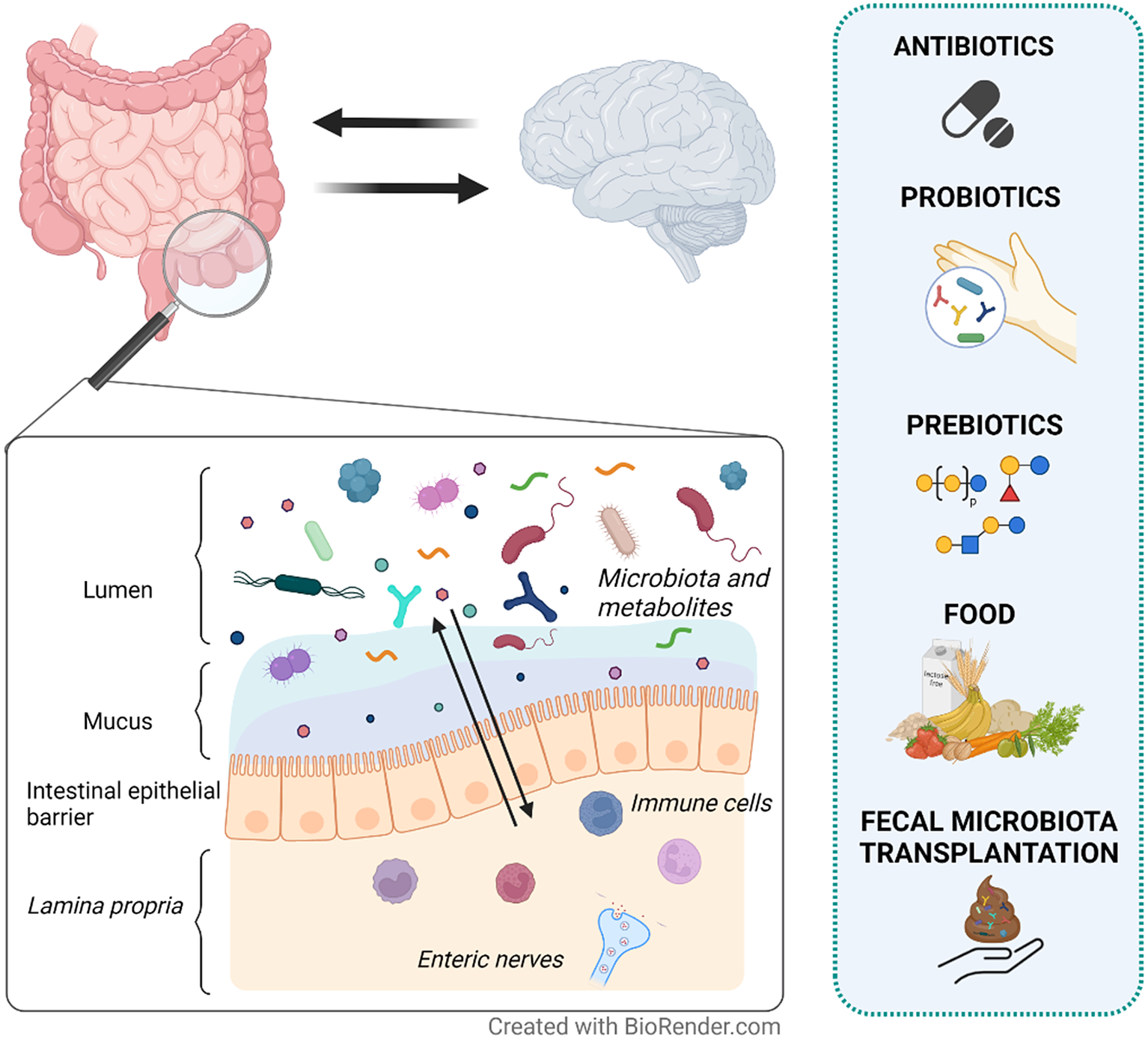
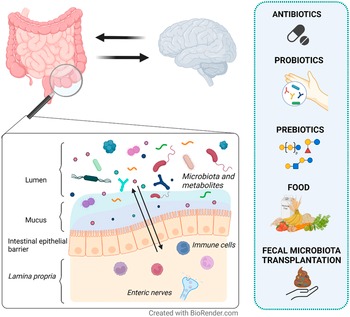
Figure 1 Therapeutic strategies proposed to modulate the gut microenvironment in patients with irritable bowel syndrome (IBS). IBS is a disorder of gut–brain interaction where alterations in either direction may influence the opposite end. The intestinal epithelial barrier separates the content of the lumen (gut microenvironment) from the underlying lamina propria. Local immune cells and enteric nerves located in the lamina propria independently or collaboratively sense and respond to signals in the gut microenvironment. Therefore, changes in the gut microenvironment are suggested to play a role in symptom generation and other factors involved in the pathophysiology of IBS. Although with mechanisms yet far from fully understood, antibiotics, probiotics, prebiotics, food and faecal microbiota transplantation (FMT) may influence the gut microenvironment (eg. microbiota and metabolites) and modulate symptoms in IBS patients.
Antibiotics
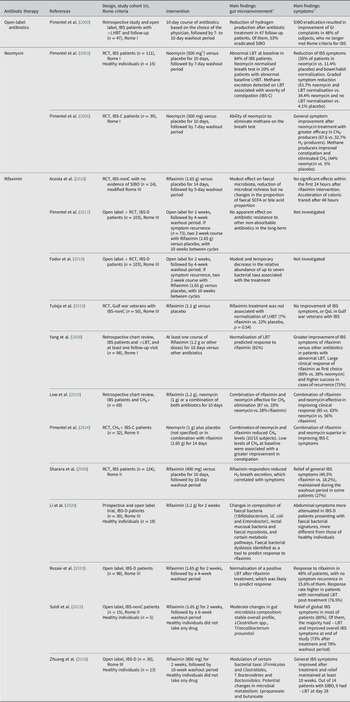
Antibiotics first showed benefits in patients with diagnosis of IBS and Small Intestinal Bacterial Overgrowth (SIBO), two entities with potentially overlapping symptoms (Pimentel et al., Reference Pimentel, Chow and Lin2000). Generally, SIBO is diagnosed using non-invasive breath tests after intake of carbohydrates, most frequently lactulose, by quantifying excreted hydrogen and methane produced by microbial fermentation (Gasbarrini et al., Reference Gasbarrini, Corazza, Gasbarrini, Montalto, Di Stefano, Basilisco, Parodi, Usai-Satta, Vernia, Anania, Astegiano, Barbara, Benini, Bonazzi, Capurso, Certo, Colecchia, Cuoco, Di Sario, Festi, Lauritano, Miceli, Nardone, Perri, Portincasa, Risicato, Sorge and Tursi2009). This test has also been suggested to reflect alterations in microbiota composition in IBS patients (Pimentel et al., Reference Pimentel, Chow and Lin2000). However, the link between IBS and SIBO is still controversial and part of the problem is related to the absence of a gold standard to define SIBO as well as poor performance of available tests (Aziz et al., Reference Aziz, Törnblom and Simrén2017). However, small bowel microbial alterations seem to be present in patients with DGBI, but are not necessarily associated with SIBO, as it is currently defined. To date, the antibiotics neomycin, rifaximin and rifamycin have been the most investigated in IBS, with potential benefits regarding improvement in IBS symptoms (Ford et al., Reference Ford, Harris, Lacy, Quigley and Moayyedi2018).
Neomycin
Little is known about the effects of the oral antibiotic neomycin on the gut microenvironment, but it has shown capacity to improve IBS symptoms and normalise the lactulose breath test (Pimentel et al., Reference Pimentel, Chow and Lin2003). In patients with IBS-C presenting with excretion of methane, neomycin administration improved symptoms along with methane being eliminated on breath test (Pimentel et al., Reference Pimentel, Chatterjee, Chow, Park and Kong2006), suggesting treatment-induced gut microbiota modulation.
Rifaximin
The influence of the non-absorbable antibiotic rifaximin on the gut microenvironment has been more extensively explored. Administration of rifaximin can negatively affect microbial richness, but did not influence the faecal SCFAs or bile acid production in IBS patients without constipation with no evidence of SIBO (Acosta et al., Reference Acosta, Camilleri, Shin, Linker Nord, O’Neill, Gray, Lueke, Donato, Burton, Szarka, Zinsmeister, Golden and Fodor2016). When SIBO is concomitant, rifaximin has been shown to lower the levels of potentially pathogenic Clostridium spp., and increase Faecalibaterium, while inducing only modest changes in the overall faecal microbiota composition. However, these changes could not be associated with symptom amelioration or normalisation of the lactulose breath test due to the lack of a notable shift in the faecal microbiota composition and also probably the analysis of faecal rather than small intestinal microbiota (Soldi et al., Reference Soldi, Vasileiadis, Uggeri, Campanale, Morelli, Fogli, Calanni, Grimaldi and Gasbarrini2015). Further, rifaximin administration in IBS-D patients seems to modify faecal microbiota composition and potentially also function, as well as eradicating SIBO (Zhuang et al., Reference Zhuang, Tian, Li, Zeng, Chen and Xiong2018). Potentially, these are the mechanisms of action underlying the relief of GI symptoms after rifaximin treatment (Zhuang et al., Reference Zhuang, Tian, Li, Zeng, Chen and Xiong2018). However, during recurrent symptoms and short-term repeated courses of rifaximin, its administration could have a short-term negative influence on certain faecal bacterial taxa (Fodor et al., Reference Fodor, Pimentel, Chey, Lembo, Golden, Israel and Carroll2019), although without apparent evidence of acquisition of antibiotic resistance in the long term (Pimentel et al., Reference Pimentel, Cash, Lembo, Wolf, Israel and Schoenfeld2017). In parallel, numerous randomised clinical trials have reported promising results in treating IBS symptoms with rifaximin (Supplementary References: Rifaximin), although with some exceptions (Tuteja et al., Reference Tuteja, Talley, Stoddard and Verne2019). Indeed, rifaximin seems to have a higher clinical response rate as primary treatment, as well as retreatment, than other antibiotics used for SIBO-IBS management. Besides, it can normalise breath test results (Soldi et al., Reference Soldi, Vasileiadis, Uggeri, Campanale, Morelli, Fogli, Calanni, Grimaldi and Gasbarrini2015; Yang et al., Reference Yang, Lee, Low, Chatterjee and Pimentel2008), especially in combination with neomycin in patients with abnormal levels of methane (Low et al., Reference Low, Hwang, Hua, Zhu, Morales and Pimentel2010; Pimentel et al., Reference Pimentel, Chang, Chua, Mirocha, DiBaise, Rao and Amichai2014), potentially reflecting changes in microbial fermentation (Sharara et al., Reference Sharara, Aoun, Abdul-Baki, Mounzer, Sidani and Elhajj2006). Interestingly, gut microbiota composition (Li et al., Reference Li, Hong, Yang, Li, Jin, Xiong, Qian and Hou2020) and breath tests (Rezaie et al., Reference Rezaie, Heimanson, McCallum and Pimentel2019) have been proposed as promising prognostic tools for treatment response, although the link between microbiota and symptoms calls for further investigations.
Rifamycin
Rifamycin SV is a poorly absorbed antibiotic that exerts its action in the distal small bowel and colon, at pH levels ≥7. To date, this antibiotic has not been associated with acquisition of multi-drug resistant bacteria (Steffen et al., Reference Steffen, Jiang, Gracias Garcia, Araujo, Stiess, Nacak, Greinwald and DuPont2018), presents in vitro proinflammatory properties (Rosette et al., Reference Rosette, Agan, Rosette, Moro, Mazzetti, Hassan and Gerloni2019), and is approved in the US for treatment of traveller’s diarrhoea (Hoy, Reference Hoy2019). New formulations of rifamycin SV are currently under development for various GI diseases. These formulations are insignificantly absorbed in healthy individuals (Di Stefano et al., Reference Di Stefano, Radicioni, Vaccani, Mazzetti, Longo and Moro2021) and demonstrate potential to improve abdominal pain and diarrhoea in IBS-D patients (Cosmo-Pharmaceuticals, 2021). More studies are still needed to decipher its effects on gut microenvironment, but, in the meantime, Rifamycin SV seems to be a promising new antibiotic therapy for the management of IBS.
Current recommendations
Hitherto, rifaximin is the only antibiotic treatment used and approved for IBS-D, but its use is not universally accepted. In the US, rifaximin is approved for 2-week treatment of patients with IBS-D and recommended in the IBS management guidelines by the American College of Gastroenterology (Lacy et al., Reference Lacy, Pimentel, Brenner, Chey, Keefer, Long and Moshiree2021). In contrast, rifaximin is currently not approved for use for IBS in Europe (Vasant et al., Reference Vasant, Paine, Black, Houghton, Everitt, Corsetti, Agrawal, Aziz, Farmer, Eugenicos, Moss-Morris, Yiannakou and Ford2021). As stated above, rifamycin SV is only approved for traveller’s diarrhoea in the US, while studies to fully support its inclusion in IBS management and its impact on gut microenvironment are awaited.
To summarise, non-absorbable antibiotics seem to be effective in the management of IBS symptoms. These antibiotics may exert their action through bactericidal effects by blocking essential biological pathways that lead to bacterial cell death (Floss and Yu, Reference Floss and Yu2005; Jana and Deb, Reference Jana and Deb2006). However, its use in IBS patients is still based on a hypothetical capacity of changing an imbalanced gut microbiota composition (Basseri et al., Reference Basseri, Weitsman, Barlow and Pimentel2011) although other not yet well-defined mechanisms might be involved (Figure 2 – based on Floss and Yu (Reference Floss and Yu2005); Jana and Deb (Reference Jana and Deb2006); Pimentel (Reference Pimentel2016) – and Table 1). More studies focusing on the mechanisms of action of antibiotics and their effect on the gut microenvironment are needed to advance our understanding of how antibiotics may be used to manage IBS.
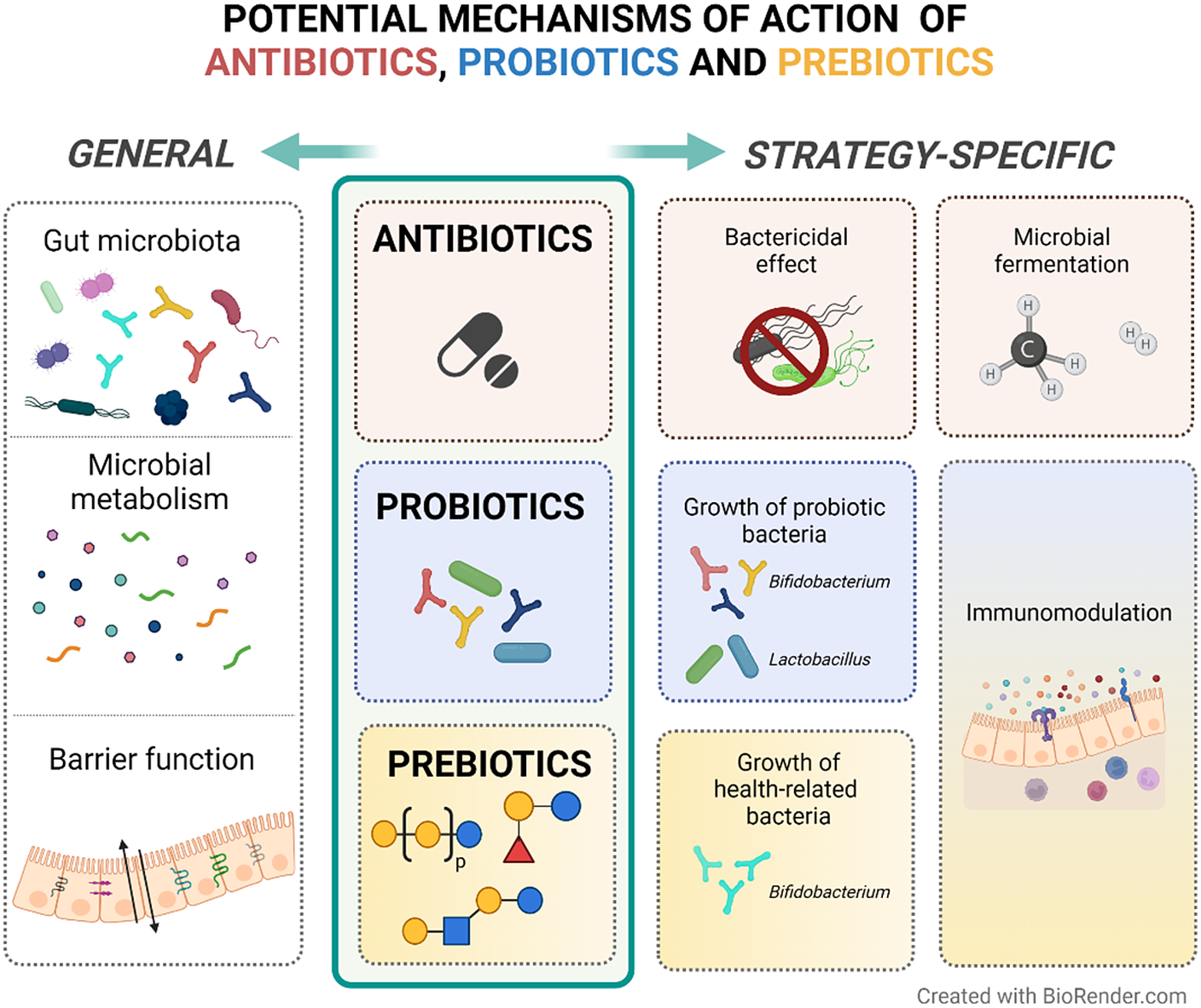
Figure 2 Potential mechanisms of action of antibiotics, probiotics and prebiotics in patients with IBS. In general, anti-, pro-, and pre-biotics are suggested to exert their therapeutic activity through favourable alterations on the gut microbiota composition, microbial metabolism products (eg. short-chain fatty acids) and gut barrier function. In particular, antibiotics can induce bacterial cell death and modulate methane and hydrogen production, which may reflect the fermentation activity of the microbiota. Both probiotics and prebiotics are suggested to have immunomodulatory effects on the host. Probiotics result in the growth of the administered bacteria, while prebiotics influence the growth of specific endogenous bacteria that are suggested to be related to health.
Box 1. Antibiotics – Key points
-
– The use of non-absorbable antibiotics in IBS is not universally approved.
-
– Clinical studies support the potential of certain non-absorbable antibiotics to improve IBS symptoms.
-
– The mechanisms involved in symptom improvement may include bactericidal effect, changes in gut microbiota composition and microbial fermentation.
Box 2. Probiotics – Key points
-
– Dependent on bacterial strains and doses, probiotics may have different mechanisms of action on gut microenvironment, including modulation of microbial composition and function, immune activity and barrier function.
-
– The large diversity of study design and bacterial strains explain gaps of knowledge and weak support of probiotics as therapeutic options in IBS.
-
– It is necessary to improve the characterisation of the mechanisms underlying the potential clinical benefits through studies using uniform study designs, approved clinical endpoints and valid mechanistic assessments.
Table 1. Overview of studies evaluating the effects of antibiotics on the gut microenvironment and clinical outcome in patients with IBS.

Abbreviations: CH4, methane; CH4+, positive-methane; H2, hydrogen; IBS, irritable bowel syndrome; IBS-C, irritable bowel syndrome with constipation; IBS-D, irritable bowel syndrome with diarrhoea; IBS-nonC, irritable bowel syndrome without constipation (=diarrhoea and mixed bowel habits); LBT, lactulose breath test (methane and hydrogen excretion); L(H)BT, lactulose (hydrogen) breath test; n, size of population (selected or randomised patients depending on study intervention); QoL, quality of life; RCT, randomised controlled trial; SCFA, short-chain fatty acid; SIBO, small intestinal bacterial overgrowth. % indicates the percentage of patients or cases. Symbols: ↑, increase; ↓, decrease, +, positive; −, negative.
a Statistically significant findings unless otherwise specified.
b Reported total daily dose.
Probiotics
Probiotics [~for life] are defined as live microorganisms that confer a health benefit on the host when administered in adequate amounts (Gibson et al., Reference Gibson, Hutkins, Sanders, Prescott, Reimer, Salminen, Scott, Stanton, Swanson, Cani, Verbeke and Reid2017). The use of probiotics for the management of GI symptoms gained popularity during the 1990s (Rolfe, Reference Rolfe2000). Here we focus on placebo-controlled studies that have investigated the effect of probiotics on gut microenvironment and their interaction with IBS symptoms. To date, studies assessing the effects of probiotics on IBS symptoms as well as published meta-analyses provide mixed results regarding the clinical efficacy of probiotic products in IBS (Ford et al., Reference Ford, Harris, Lacy, Quigley and Moayyedi2018; McFarland et al., Reference McFarland, Karakan and Karatas2021).
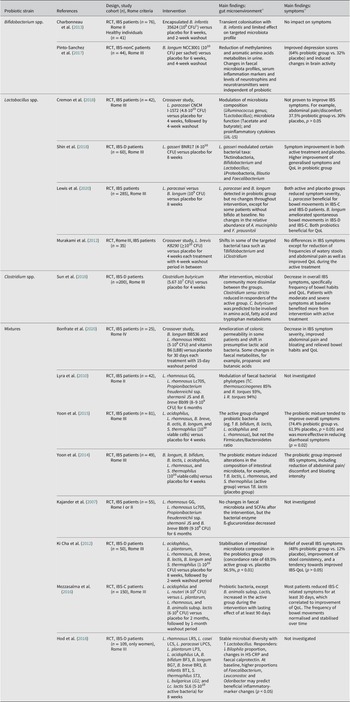
Bifidobacterium spp. and Lactobacillus spp.
Bifidobacteria is generally associated with health benefits throughout life, although its abundance varies with age (O’Callaghan and van Sinderen, Reference O’Callaghan and van Sinderen2016). Low levels of bifidobacteria in IBS (Pittayanon et al., Reference Pittayanon, Lau, Yuan, Leontiadis, Tse, Surette and Moayyedi2019) may be improved after administration of probiotic bifidobacteria and successfully alleviate symptoms (Ford et al., Reference Ford, Harris, Lacy, Quigley and Moayyedi2018; Pinto-Sanchez et al., Reference Pinto-Sanchez, Hall, Ghajar, Nardelli, Bolino, Lau, Martin, Cominetti, Welsh, Rieder, Traynor, Gregory, De Palma, Pigrau, Ford, Macri, Berger, Bergonzelli, Surette, Collins, Moayyedi and Bercik2017). However, the link between the clinical benefit and the impact on the gut microenvironment after bifidobacteria supplementation needs further investigations (Table 2).
Box 3. Prebiotics – Key points
-
– The clinical benefit is dependent on prebiotic type and dose.
-
– Suggested mechanisms of action of prebiotics include increase of growth and activity/function of health-associated bacteria.
-
– Subgroup analyses and assessments of the effect of different doses are required to better understand the therapeutic potential of prebiotics in IBS.
Table 2. Overview of studies evaluating the effects of probiotics on the gut microenvironment and clinical outcome in patients with IBS.

Abbreviations: B, Bifidobacterium; C, Clostridium; CFU, colony-forming units; HS‐CRP high sensitivity C-Reactive Protein; IBS, irritable bowel syndrome; IBS-C, irritable bowel syndrome with constipation; IBS-D, irritable bowel syndrome with diarrhoea; QoL, quality of life; L., Lactobacillus; Lc, Lactococcus; RCT, randomised controlled trial; S., Streptococcus. % indicates the percentage of patients or cases. Symbols: ↑, increase; ↓, decrease.
a Statistically significant findings unless otherwise specified.
b Reported total daily dose.
Hitherto, there is no consensus concerning the potential role of Lactobacillus in IBS patients (Liu et al., Reference Liu, Wu, Chen, Chen, Shen and Liu2017; Pittayanon et al., Reference Pittayanon, Lau, Yuan, Leontiadis, Tse, Surette and Moayyedi2019). Still, the probiotic Lactobacillus is generally considered beneficial (Heeney et al., Reference Heeney, Gareau and Marco2018) and its supplementation in IBS patients seems promising for managing their symptoms (Ford et al., Reference Ford, Harris, Lacy, Quigley and Moayyedi2018; Murakami et al., Reference Murakami, Habukawa, Nobuta, Moriguchi and Takemura2012). It has been suggested that Lactobacillus modulates specific bacterial taxa (Cremon et al., Reference Cremon, Guglielmetti, Gargari, Taverniti, Castellazzi, Valsecchi, Tagliacarne, Fiore, Bellini, Bertani, Gambaccini, Cicala, Germanà, Vecchi, Pagano, Barbaro, Bellacosa, Stanghellini and Barbara2018; Murakami et al., Reference Murakami, Habukawa, Nobuta, Moriguchi and Takemura2012), alters the levels of faecal SCFAs or pro-inflammatory cytokines (Cremon et al., Reference Cremon, Guglielmetti, Gargari, Taverniti, Castellazzi, Valsecchi, Tagliacarne, Fiore, Bellini, Bertani, Gambaccini, Cicala, Germanà, Vecchi, Pagano, Barbaro, Bellacosa, Stanghellini and Barbara2018; Shin et al., Reference Shin, Choi, Kim, Hong, Park, Kim, Kwon, Lee and Hahm2018), and could potentially be associated with improvement of IBS symptoms (Table 2).
Box 4. Food and dietary habits – Key points
-
– Modifying the dietary habits constitutes an accessible and, to a large extent, safe strategy for IBS management, even though more extreme diets may be unfavourable for gut microbiota composition and function.
-
– Dietary habits impact and interact with the gut microbiota.
-
– The existing literature suggests that the management of IBS symptoms through dietary interventions may potentially involve modulation of gut microbiota composition, microbial metabolites, immune activity and intestinal permeability.
-
– Further studies evaluating the short- and long-term impact of dietary habits on the gut microenvironment are needed.
Lactobacillus and Bifidobacterium may differ in their effect on modulating microbiota composition (Table 2; Supplementary References: Bifidobacterium spp. and Lactobacillus spp.). Overall, bifidobacteria show a greater tendency towards improvement of global IBS symptoms and pain scores (Ford et al., Reference Ford, Harris, Lacy, Quigley and Moayyedi2018), although lactobacilli may have a similar efficacy (Lewis et al., Reference Lewis, Antony, Crowley, Piano, Bhardwaj, Tompkins and Evans2020).
Box 5. FMT – Key points
-
– FMT application in IBS is still constrained to clinical research due to limitations in safety and partly divergent efficacy data.
-
– Donors with a favourable microbiota (ie. diverse and stable) seem to be important for FMT success.
-
– The poor link between microbiota alterations and symptom improvements suggests the involvement of other mechanisms of action that are yet to be clarified.
-
– FMT shows a potential as a therapy for IBS, however, optimization of the protocol (eg. delivery route, dose and frequency) is required to move forward with clinical usage.
Other probiotic bacteria and mixtures
Although the clinical potential of other single-strain probiotic bacteria has been investigated, the potential mechanisms of action are still insufficiently investigated (Ford et al., Reference Ford, Harris, Lacy, Quigley and Moayyedi2018). Clostridium butyricum, however, is one of very few exceptions. After 4-week supplementation, Clostridium butyricum shifts the microbiota composition, as well as potentially modulates the metabolic pathways of amino acids, fatty acids and tryptophan in patients with IBS. Further, this probiotic may provide a greater benefit to patients with moderate and severe symptoms, and may alleviate overall IBS symptoms (Sun et al., Reference Sun, Li, Li, Li, Zhai, Wang, Yang, Gu, Song, Li, Zuo and Li2018).
Probiotic mixtures may possibly have better efficacy than single-strain probiotics in IBS patients (Ford et al., Reference Ford, Harris, Lacy, Quigley and Moayyedi2018). Multiple formulations containing different combinations of Lactobacillus and Bifidobacterium strains as well as other bacterial genera have been tested in patients with IBS. Such formulations seem to have multiple effects on gut microenvironment, that is, shifting the microbiota composition (Bonfrate et al., Reference Bonfrate, Di Palo, Celano, Albert, Vitellio, De Angelis, Gobbetti and Portincasa2020; Lyra et al., Reference Lyra, Krogius-Kurikka, Nikkilä, Malinen, Kajander, Kurikka, Korpela and Palva2010; Yoon et al., Reference Yoon, Sohn, Lee, Lee, Lee, Jun, Lee, Yoon, Choi, Chung and Seo2014, Reference Yoon, Park, Lee, Seo, Shin and Kim2015), ameliorating the colonic permeability (Bonfrate et al., Reference Bonfrate, Di Palo, Celano, Albert, Vitellio, De Angelis, Gobbetti and Portincasa2020), or changing the bacterial enzyme ß-glucuronidase activity (Kajander et al., Reference Kajander, Krogius-Kurikka, Rinttilä, Karjalainen, Palva and and Korpela2007), although not all studies are in agreement (Kajander et al., Reference Kajander, Krogius-Kurikka, Rinttilä, Karjalainen, Palva and and Korpela2007; Ki Cha et al., Reference Ki Cha, Mun Jung, Hwan Choi, Song, Woong Lee, Joon Kim, Hyuk, Kyung Chang, Kim, Chung and Seo2012; Table 2). These mixtures may be effective in improving IBS symptoms and the severity of the disorder (Bonfrate et al., Reference Bonfrate, Di Palo, Celano, Albert, Vitellio, De Angelis, Gobbetti and Portincasa2020; Yoon et al., Reference Yoon, Sohn, Lee, Lee, Lee, Jun, Lee, Yoon, Choi, Chung and Seo2014, Reference Yoon, Park, Lee, Seo, Shin and Kim2015; Supplementary References: Mixtures) in certain IBS subtypes (Hod et al., Reference Hod, Dekel, Aviv Cohen, Sperber, Ron, Boaz, Berliner and Maharshak2018; Ki Cha et al., Reference Ki Cha, Mun Jung, Hwan Choi, Song, Woong Lee, Joon Kim, Hyuk, Kyung Chang, Kim, Chung and Seo2012; Mezzasalma et al., Reference Mezzasalma, Manfrini, Ferri, Sandionigi, La Ferla, Schiano, Michelotti, Nobile, Labra and Di Gennaro2016) In addition, the response to the probiotic formulation may be predicted by faecal bacterial patterns at baseline (Hod et al., Reference Hod, Dekel, Aviv Cohen, Sperber, Ron, Boaz, Berliner and Maharshak2018; Table 2). Further, probiotic mixtures might also have a long-term effect on symptoms or specific bacterial taxa even after the supplement removal (Mezzasalma et al., Reference Mezzasalma, Manfrini, Ferri, Sandionigi, La Ferla, Schiano, Michelotti, Nobile, Labra and Di Gennaro2016), although little is known about these effects.
Current recommendations
While all these results are encouraging, the quality of evidence for IBS management is still poor and the general recommendation regarding the use of probiotics in clinical practice guidelines is weak. This reality is reflected in the recommendations in recent guidelines. In general, large professional societies recommend against widespread routine use of probiotics or avoid recommending specific products due to the weak supporting literature. Still, it is acknowledged that probiotics may be useful in select patients and that patient preference is important in the final decision of treatment regimen (Lacy et al., Reference Lacy, Pimentel, Brenner, Chey, Keefer, Long and Moshiree2021; Su et al., Reference Su, Ko, Bercik, Falck-Ytter, Sultan, Weizman and Morgan2020; Vasant et al., Reference Vasant, Paine, Black, Houghton, Everitt, Corsetti, Agrawal, Aziz, Farmer, Eugenicos, Moss-Morris, Yiannakou and Ford2021). For this reason, further research with a higher degree of consensus concerning study design and bacterial strains may help fill the current knowledge gaps and improve the characterisation of the mechanisms of action that have so far been suggested (Figure 2).
Prebiotics
Prebiotics, unlike probiotics, are not microorganisms but substrates that are selectively metabolised by health-promoting microorganisms (Gibson et al., Reference Gibson, Hutkins, Sanders, Prescott, Reimer, Salminen, Scott, Stanton, Swanson, Cani, Verbeke and Reid2017). Prebiotics, directly or through cross-feeding, enhance the activity (eg. SCFAs production) or growth of health-associated bacteria (eg. bifidobacteria and lactobacilli), without aggravating adverse effects such as distension due to gas production (Gibson et al., Reference Gibson, Hutkins, Sanders, Prescott, Reimer, Salminen, Scott, Stanton, Swanson, Cani, Verbeke and Reid2017). Since the first definition of prebiotics in the 90s, studies assessing the direct effect of prebiotics in IBS patients have been limited (Table 3) and with sparse but still potential clinical benefits in IBS (Ford et al., Reference Ford, Harris, Lacy, Quigley and Moayyedi2018).
Table 3. Overview of studies evaluating the effects of prebiotics on the gut microenvironment and clinical outcome in patients with IBS.
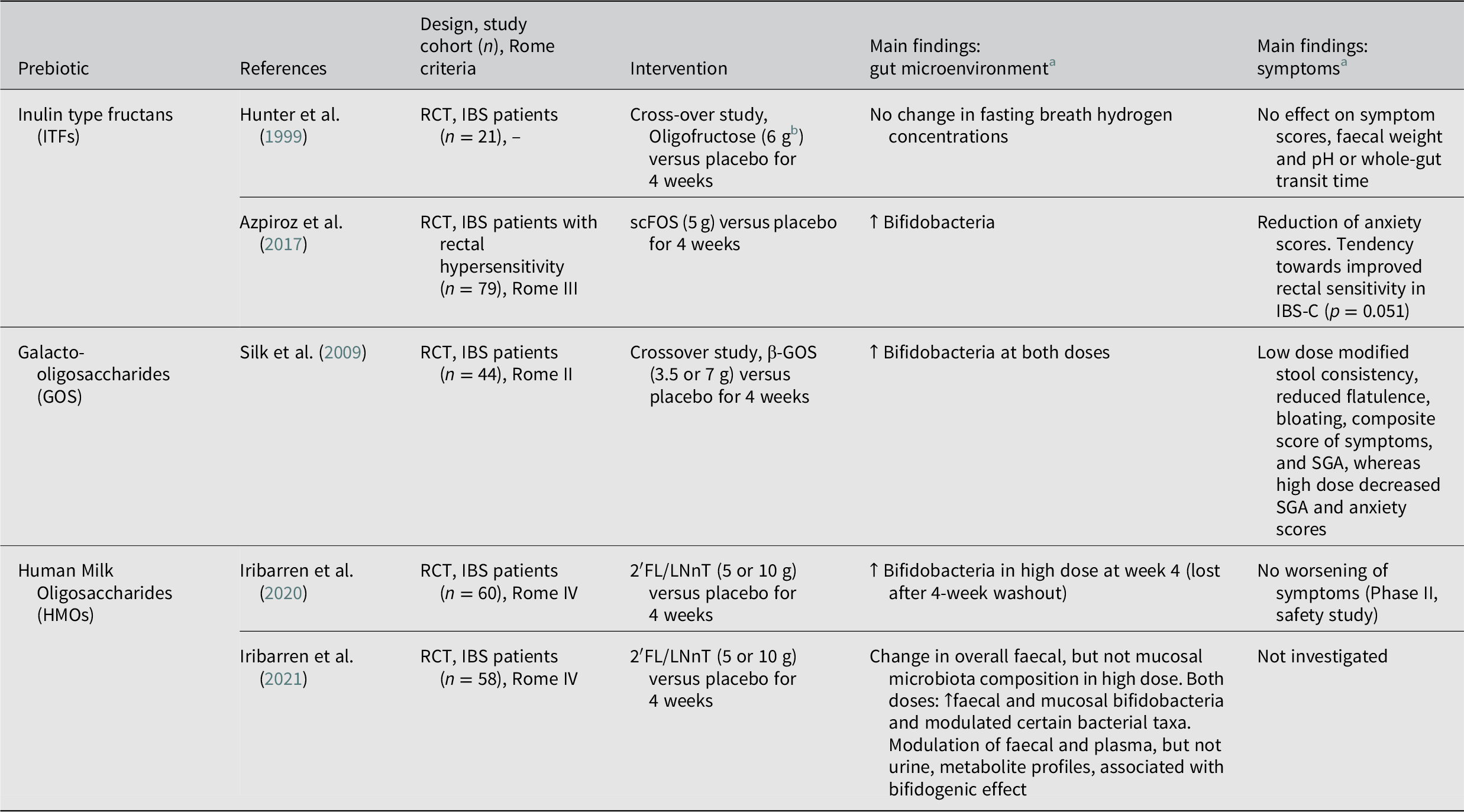
Abbreviations: FOS, fructooligosaccharide; GOS, galactooligosaccharide; HMO, Human Milk Oligosaccharide; 2′FL/LNnT, 4:1 mix of 2′-fucosyllactose, and lacto-N-neotetraose; IBS, irritable bowel syndrome; IBS-C, irritable bowel syndrome with constipation; IBS-D, irritable bowel syndrome with diarrhoea; QoL, quality of life; scFOS; short-chain fructooligosaccharide; SGA, subjective global assessment; RCT, randomised controlled trial. Symbols: ↑, increase; ↓, decrease.
a Statistically significant findings unless otherwise specified.
b Reported total daily dose.
Inulin type fructans
Inulin type fructans (ITFs) is a combined term for inulin, fructooligosaccharides (FOS) and oligofructose and refers to non-digestible, linear fructans, which can be found naturally in vegetables and fruits (Wilson and Whelan, Reference Wilson and Whelan2017). Hunter et al. (Reference Hunter, Tuffnell and Lee1999) assessed the therapeutic effect of oligofructose (6 g/day) in IBS patients, in a 4-week crossover study, finding no modifications on symptom severity or fasting breathe hydrogen concentrations. Further, while high levels of FOS intake (20 g/day) caused transient worsening of symptoms (Olesen and Gudmand-Hoyer, Reference Olesen and Gudmand-Hoyer2000), a lower dose of short-chain fructooligosaccharides (scFOS) (5 g/day) was well tolerated in IBS patients with rectal hypersensitivity and led to significantly increased faecal bifidobacteria counts and decreased anxiety scores (Azpiroz et al., Reference Azpiroz, Dubray, Bernalier-Donadille, Cardot, Accarino, Serra, Wagner, Respondek and Dapoigny2017). In this study, however, improvement in symptom severity was similar to placebo (Azpiroz et al., Reference Azpiroz, Dubray, Bernalier-Donadille, Cardot, Accarino, Serra, Wagner, Respondek and Dapoigny2017).
Galactooligosaccharides
β-galactooligosaccharides (β-GOS) are non-digestible oligosaccharides synthesised from lactose using microbial β-galactosidases, and unlike other GOS naturally found in plants they are selectively fermented by bacteria (Wilson and Whelan, Reference Wilson and Whelan2017). A crossover pilot trial by Silk et al. (Reference Silk, Davis, Vulevic, Tzortzis and Gibson2009) showed that 4-weeks of β-GOS treatment at both 3.5 and 7 g/day relieved symptoms and, dose dependently, increased faecal bifidobacteria. In this study, the lower dose led to decreased flatulence and bloating, while the higher dose resulted in increased bloating but improved anxiety scores (Silk et al., Reference Silk, Davis, Vulevic, Tzortzis and Gibson2009).
Human milk oligosaccharides
Human breast milk contains high concentrations of structurally diverse glycans, collectively referred to as human milk oligosaccharides (HMOs). HMOs are non-digestible oligosaccharides, that are selectively metabolised by bacteria (eg. Bifidobacterium longum subsp. infantis), and beneficially influence infant gut health, and are considered as the first prebiotics new-borns encounter (Bode, Reference Bode2012). However, studies on the prebiotic activity and health benefit of HMOs in adults are few. Recently our group conducted two studies on the impact of 4:1 HMO mix containing 2′-O-fucosyllactose and lacto-N-neotetraose (2´FL/LNnT) in IBS patients (Iribarren et al., Reference Iribarren, Törnblom, Aziz, Magnusson, Sundin, Vigsnaes, Amundsen, McConnell, Seitzberg, Öhman and Simrén2020, Reference Iribarren, Magnusson, Vigsnæs, Aziz, Amundsen, Šuligoj, Juge, Patel, Sapnara, Johnsen, Sørensen, Sundin, Törnblom, Simrén and Öhman2021). Our findings show that adult patients tolerate 2′FL/LNnT supplementation, regardless of the dose (5 or 10 g/day) (Iribarren et al., Reference Iribarren, Törnblom, Aziz, Magnusson, Sundin, Vigsnaes, Amundsen, McConnell, Seitzberg, Öhman and Simrén2020). Further, a potential change of the gut microenvironment was supported by modulation of the gut microbiota composition (Iribarren et al., Reference Iribarren, Törnblom, Aziz, Magnusson, Sundin, Vigsnaes, Amundsen, McConnell, Seitzberg, Öhman and Simrén2020, Reference Iribarren, Magnusson, Vigsnæs, Aziz, Amundsen, Šuligoj, Juge, Patel, Sapnara, Johnsen, Sørensen, Sundin, Törnblom, Simrén and Öhman2021) and faecal and plasma metabolite profiles (Iribarren et al., Reference Iribarren, Magnusson, Vigsnæs, Aziz, Amundsen, Šuligoj, Juge, Patel, Sapnara, Johnsen, Sørensen, Sundin, Törnblom, Simrén and Öhman2021). When administered at 5 g/day for 12 weeks, the same 2′FL/LNnT formulation led to normalisation of stool forms and improvements in abdominal pain, bloating, overall IBS and quality of life scores in all IBS subgroups in a multicentre, placebo uncontrolled, open label trial (Palsson et al., Reference Palsson, Peery, Seitzberg, Amundsen, McConnell and Simrén2020). However, given the large placebo effect on IBS symptoms, a placebo-controlled study is essential to confirm the clinical efficacy of HMOs in IBS.
Current recommendations
According to the International Scientific Association for Probiotics and Prebiotics (ISAPP), ITFs and β-GOS are the only accepted prebiotics, while HMOs are promising candidates (Gibson et al., Reference Gibson, Hutkins, Sanders, Prescott, Reimer, Salminen, Scott, Stanton, Swanson, Cani, Verbeke and Reid2017). Overall, despite the bifidogenic effect reported in placebo-controlled studies, prebiotics do not seem to convincingly improve IBS symptoms or quality of life in IBS patients (Wilson et al., Reference Wilson, Rossi, Dimidi and Whelan2019; Table 3). Furthermore, due to high fermentability followed by gas production, high doses of prebiotics entail a risk to exacerbate symptoms in IBS patients (Muir, Reference Muir2019). Yet, given the specific mechanistic effect of each prebiotic type, subgroup analyses as well as assessments of different doses of prebiotics may provide a more optimistic view of their potential to improve symptoms in IBS patients.
Food and dietary habits
A large majority of IBS patients report generation and aggravation of GI symptoms following meal ingestion (Böhn et al., Reference Böhn, Störsrud, Törnblom, Bengtsson and Simrén2013). These symptoms are potentially triggered via primary (eg. prebiotic and osmotic effects) or secondary effects (eg. intraluminal pH and microbiome effects) (Spencer et al., Reference Spencer, Chey and Eswaran2014). Furthermore, local allergy-like reactions in the gut have recently been proposed to be of importance in subsets of patients (Aguilera-Lizarraga et al., Reference Aguilera-Lizarraga, Florens, Viola, Jain, Decraecker, Appeltans, Cuende-Estevez, Fabre, Van Beek, Perna, Balemans, Stakenborg, Theofanous, Bosmans, Mondelaers, Matteoli, Ibiza Martínez, Lopez-Lopez, Jaramillo-Polanco, Talavera, Alpizar, Feyerabend, Rodewald, Farre, Redegeld, Si, Raes, Breynaert, Schrijvers, Bosteels, Lambrecht, Boyd, Hoh, Cabooter, Nelis, Augustijns, Hendrix, Strid, Bisschops, Reed, Vanner, Denadai-Souza, Wouters and Boeckxstaens2021; Fritscher-Ravens et al., Reference Fritscher-Ravens, Pflaum, Mösinger, Ruchay, Röcken, Milla, Das, Böttner, Wedel and Schuppan2019). Patients commonly find food rich in carbohydrates and fat, dairy products or gluten as aggravating (Böhn et al., Reference Böhn, Störsrud, Törnblom, Bengtsson and Simrén2013). Thus, the exclusion or reduction of such food items from the diet is a regular practice to reduce symptoms (Lenhart et al., Reference Lenhart, Dong, Joshi, Jaffe, Choo, Liu, Jacobs, Lagishetty, Shih, Labus, Gupta, Tillisch, Mayer and Chang2022), but comes with the risk of severe food avoidance and restriction in a proportion of IBS patients (Melchior et al., Reference Melchior, Algera, Colomier, Törnblom, Simrén and Störsrud2022). This habit may also negatively impact the gut microbiota (Altomare et al., Reference Altomare, Del Chierico, Rocchi, Emerenziani, Nuglio, Putignani, Angeletti, Lo Presti, Ciccozzi, Russo, Cocca, Ribolsi, Muscaritoli, Cicala and Guarino2021; Lenhart et al., Reference Lenhart, Dong, Joshi, Jaffe, Choo, Liu, Jacobs, Lagishetty, Shih, Labus, Gupta, Tillisch, Mayer and Chang2022), reduce the quality of the diet (Altomare et al., Reference Altomare, Del Chierico, Rocchi, Emerenziani, Nuglio, Putignani, Angeletti, Lo Presti, Ciccozzi, Russo, Cocca, Ribolsi, Muscaritoli, Cicala and Guarino2021), and the overall nutrient intake (Tigchelaar et al., Reference Tigchelaar, Mujagic, Zhernakova, Hesselink, Meijboom, Perenboom, Masclee, Wijmenga, Feskens and Jonkers2017). Hence, both positive and negative effects of dietary interventions and restrictions in IBS exist.
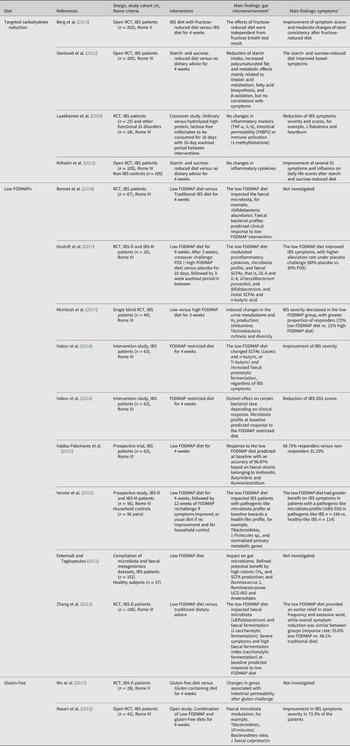
Targeted carbohydrate reduction diet
Impaired absorption of short-chain (eg. fructose, sorbitol, lactose and sucrose) and long-chain (eg. starch) carbohydrates by the small intestine may contribute to GI symptoms (Hasler, Reference Hasler2006; Yao et al., Reference Yao, Tan, van Langenberg, Barrett, Rose, Liels, Gibson and Muir2014). The generation of symptoms may be explained by an altered microbial fermentation and carbohydrate metabolism (Yao et al., Reference Yao, Tan, van Langenberg, Barrett, Rose, Liels, Gibson and Muir2014) which can potentially be identified using breath tests (Gasbarrini et al., Reference Gasbarrini, Corazza, Gasbarrini, Montalto, Di Stefano, Basilisco, Parodi, Usai-Satta, Vernia, Anania, Astegiano, Barbara, Benini, Bonazzi, Capurso, Certo, Colecchia, Cuoco, Di Sario, Festi, Lauritano, Miceli, Nardone, Perri, Portincasa, Risicato, Sorge and Tursi2009; Table 4). Hence, the elimination or reduction of specific carbohydrates may improve IBS symptoms (Berg et al., Reference Berg, Fagerli, Martinussen, Myhre, Florholmen and Goll2013; Supplementary References: Targeted carbohydrate reduction) and modulate metabolomic pathways (Stenlund et al., Reference Stenlund, Nilholm, Chorell, Roth, D’Amato and Ohlsson2021), but there is not yet any evidence for effect on inflammatory parameters (Nilholm et al., Reference Nilholm, Larsson, Sonestedt, Roth and Ohlsson2021). Nonetheless, clinical benefit may not always correlate with breath test results as a measure of microbial fermentation, so the exact mechanisms underlying symptom improvement with targeted carbohydrate reduction diets are still partly unresolved (Berg et al., Reference Berg, Fagerli, Martinussen, Myhre, Florholmen and Goll2013; Yao et al., Reference Yao, Tan, van Langenberg, Barrett, Rose, Liels, Gibson and Muir2014; Table 4).
Table 4. Overview of studies evaluating the effects of food on the gut microenvironment and clinical outcome in patients with IBS.

Abbreviations: CH4, methane; FABP2, Fatty Acid Binding Protein 2; FODMAP, Fermentable, Oligosaccharides, Disaccharides, Mono-saccharides, And Polyols; FOS, fructo-oligosaccharides; GI, gastrointestinal; H2, hydrogen; IL, interleukin; IBS, irritable bowel syndrome; IBS-C, irritable bowel syndrome with constipation; IBS-D, irritable bowel syndrome with diarrhoea; IBS-M, irritable bowel syndrome with mixed bowel habits; IBS-SSS, irritable bowel syndrome-severity score system; RCT, randomised controlled trial; SCFA, short-chain fatty acid; TNF-α, tumour necrosis factor alpha. % indicates the percentage of patients or cases. Symbols: ↑, increase; ↓, decrease.
a Statistically significant findings unless otherwise specified.
Low FODMAP diet
The term Fermentable Oligo-, Di- and Mono-saccharides And Polyols (FODMAPs) was introduced by Gibson and Shepherd (Reference Gibson and Shepherd2005). These dietary FODMAPs are poorly absorbed short-chain carbohydrates that reach the distal parts of the small intestine and the large intestine intact (Gibson and Shepherd, Reference Gibson and Shepherd2005). There, they constitute a source of nutrients for specific microbial species (McIntosh et al., Reference McIntosh, Reed, Schneider, Dang, Keshteli, De Palma, Madsen, Bercik and Vanner2017) and are fermented. As a result, gases, including hydrogen, methane and carbon dioxide, may be produced which together with increased intestinal water content via osmotic effects of the luminal carbohydrates, cause intestinal distension triggering GI symptoms. Therefore, reduction of foods rich in certain short-chain carbohydrates intake can impact hydrogen and methane production and luminal distension (Staudacher and Whelan, Reference Staudacher and Whelan2017).
Since the first retrospective study investigating the potential of a low FODMAP diet in patients with IBS and fructose malabsorption (Shepherd and Gibson, Reference Shepherd and Gibson2006), multiple studies have followed and increased the evidence-base for efficacy of the low FODMAP diet as a strategy for managing IBS (Black et al., Reference Black, Staudacher and Ford2021; Supplementary References: Low FODMAP diet). The positive symptomatic effect of reducing these carbohydrates may, however, in parallel negatively influence the colonic luminal microenvironment by altering gut microbiota composition and reduce bifidobacteria abundance, as well as other health-associated gut bacteria (Bennet et al., Reference Bennet, Böhn, Störsrud, Liljebo, Collin, Lindfors, Törnblom, Öhman and Simrén2018; McIntosh et al., Reference McIntosh, Reed, Schneider, Dang, Keshteli, De Palma, Madsen, Bercik and Vanner2017; Table 4). Interestingly, the clinical response to a low FODMAP diet may be predicted by gut microbiota composition before the start of the dietary intervention (Bennet et al., Reference Bennet, Böhn, Störsrud, Liljebo, Collin, Lindfors, Törnblom, Öhman and Simrén2018; Valdez-Palomares et al., Reference Valdez-Palomares, Nambo-Venegas, Uribe-García, Mendoza-Vargas, Granados-Portillo, Meraz-Cruz and Palacios-González2021; Valeur et al., Reference Valeur, Småstuen, Knudsen, Lied and Røseth2018), by high colonic methane and SCFA production (Eetemadi and Tagkopoulos, Reference Eetemadi and Tagkopoulos2021), and by high saccharolytic fermentation activity (Zhang et al., Reference Zhang, Feng, Wang, Fox, Luo, Du, Chen, Chen, He, Zhu, Hu, Chen, Long, Zhu, Xu, Deng, Misselwitz, Lang, Yilmaz, Kim, Owyang and Dai2021), supporting the importance of the gut microenvironment. However, not all studies have demonstrated a correlation between fermentation or gut microbiota profile and IBS symptoms (Table 4) or show substantial benefits in symptomatology (Nordin et al., Reference Nordin, Brunius, Landberg and Hellström2022). In addition, even though a low FODMAP diet or β-GOS supplementation for 4 weeks have very similar effects on symptom improvement, discontinuation of a low FODMAP diet may lead to a faster reappearance of symptoms as compared to elimination of prebiotic β-GOS supplementation. This suggests that the effect of a low FODMAP diet on symptoms may be mediated through the food and its interactions with the microbiota, rather than a direct effect on microbiota composition (Huaman et al., Reference Huaman, Mego, Manichanh, Cañellas, Cañueto, Segurola, Jansana, Malagelada, Accarino, Vulevic, Tzortzis, Gibson, Saperas, Guarner and Azpiroz2018; Simrén, Reference Simrén2018).
The long-term efficacy and safety of the reduction of FODMAP intake are still surrounded by uncertainties. Even so, a low FODMAP diet seems to have a sustained effect on IBS symptoms and quality of life over at least 6 months and with a high rate of adherence (Supplementary References: Long-term low FODMAP diet). Nevertheless, to date, a long-term restrictive low FODMAP diet is not considered to be beneficial because it can result in nutritional deficiencies (Harvie et al., Reference Harvie, Chisholm, Bisanz, Burton, Herbison, Schultz and Schultz2017) and it can negatively influence the gut microenvironment (Bennet et al., Reference Bennet, Böhn, Störsrud, Liljebo, Collin, Lindfors, Törnblom, Öhman and Simrén2018; McIntosh et al., Reference McIntosh, Reed, Schneider, Dang, Keshteli, De Palma, Madsen, Bercik and Vanner2017). Although few long-term studies exist, two studies support that a modified low FODMAP diet can be nutritionally adequate up to 18 months, without adversely impacting on food-related quality of life (O’Keeffe et al., Reference O’Keeffe, Jansen, Martin, Williams, Seamark, Staudacher, Irving, Whelan and Lomer2018), but may reduce diet quality (Staudacher et al., Reference Staudacher, Ralph, Irving, Whelan and Lomer2020). Consequently, following a period of strict FODMAP exclusion, a gradual reintroduction of selected FODMAP is recommended to identify tolerable long-term solutions and increase dietary variety and nutrients (Harvie et al., Reference Harvie, Chisholm, Bisanz, Burton, Herbison, Schultz and Schultz2017).
Gluten/wheat-free diet
Gluten is a complex, water-soluble protein found in grains. Following gluten intake, some IBS patients may report GI and extra-intestinal symptoms such as fatigue, similar to patients with coeliac disease, even though intestinal structural alterations are absent (Biesiekierski et al., Reference Biesiekierski, Newnham, Irving, Barrett, Haines, Doecke, Shepherd, Muir and Gibson2011). This entity is known as non-coeliac gluten/wheat intolerance and can overlap with IBS (Spencer et al., Reference Spencer, Chey and Eswaran2014). The exclusion of gluten from the diet has been shown to successfully improve IBS symptoms in subgroups of patients (Supplementary References: Gluten-free diet), but not all studies agree with this (Nordin et al., Reference Nordin, Brunius, Landberg and Hellström2022). Interestingly, one study demonstrated that approximately one third of IBS patients who benefit from a gluten-free diet suffer from wheat sensitivity (Barmeyer et al., Reference Barmeyer, Schumann, Meyer, Zielinski, Zuberbier, Siegmund, Schulzke, Daum and Ullrich2017), which may be triggered by high levels of the FODMAP fructan found in wheat (Spencer et al., Reference Spencer, Chey and Eswaran2014). The relevance of fructan, rather than gluten, for symptom generation in these patients was also supported by a recent randomised controlled challenge study (Skodje et al., Reference Skodje, Sarna, Minelle, Rolfsen, Muir, Gibson, Veierød, Henriksen and Lundin2018). Hence, gluten may not be the unique offensive factor (Biesiekierski et al., Reference Biesiekierski, Peters, Newnham, Rosella, Muir and Gibson2013) and it is currently often wheat, rather than gluten per se, that is considered to be linked to IBS (Zannini and Arendt, Reference Zannini and Arendt2018).
So far, the vast knowledge about the effect of gluten/wheat restricted diets on the gut microenvironment is obtained by studies on healthy individuals. These studies have described the ability of a gluten-free diet to shape the gut microbiota composition (Bonder et al., Reference Bonder, Tigchelaar, Cai, Trynka, Cenit, Hrdlickova, Zhong, Vatanen, Gevers, Wijmenga, Wang and Zhernakova2016; De Palma et al., Reference De Palma, Nadal, Collado and Sanz2009; Hansen et al., Reference Hansen, Roager, Søndertoft, Gøbel, Kristensen, Vallès-Colomer, Vieira-Silva, Ibrügger, Lind, Mærkedahl, Bahl, Madsen, Havelund, Falony, Tetens, Nielsen, Allin, Frandsen, Hartmann, Holst, Sparholt, Holck, Blennow, Moll, Meyer, Hoppe, Poulsen, Carvalho, Sagnelli, Dalgaard, Christensen, Lydolph, Ross, Villas-Bôas, Brix, Sicheritz-Pontén, Buschard, Linneberg, Rumessen, Ekstrøm, Ritz, Kristiansen, Nielsen, Vestergaard, Færgeman, Raes, Frøkiær, Hansen, Lauritzen, Gupta, Licht and Pedersen2018; Pinto-Sanchez et al., Reference Pinto-Sanchez, Nardelli, Borojevic, De Palma, Calo, McCarville, Caminero, Basra, Mordhorst, Ignatova, Hansen, Uhde, Norman, Murray, Smecuol, Armstrong, Bai, Schuppan, Collins, Alaedini, Moayyedi, Verdu and Bercik2020) and modulate activity levels of bacterial metabolic pathways (Bonder et al., Reference Bonder, Tigchelaar, Cai, Trynka, Cenit, Hrdlickova, Zhong, Vatanen, Gevers, Wijmenga, Wang and Zhernakova2016). Additionally, a low gluten diet seems to induce moderate changes in the gut microbiota (eg. decreasing bifidobacteria), microbial function and host physiology biomarkers in healthy individuals. All these changes are thought to lead to relative improvements of GI symptoms (eg. bloating) although the role of gluten per se is still uncertain (Hansen et al., Reference Hansen, Roager, Søndertoft, Gøbel, Kristensen, Vallès-Colomer, Vieira-Silva, Ibrügger, Lind, Mærkedahl, Bahl, Madsen, Havelund, Falony, Tetens, Nielsen, Allin, Frandsen, Hartmann, Holst, Sparholt, Holck, Blennow, Moll, Meyer, Hoppe, Poulsen, Carvalho, Sagnelli, Dalgaard, Christensen, Lydolph, Ross, Villas-Bôas, Brix, Sicheritz-Pontén, Buschard, Linneberg, Rumessen, Ekstrøm, Ritz, Kristiansen, Nielsen, Vestergaard, Færgeman, Raes, Frøkiær, Hansen, Lauritzen, Gupta, Licht and Pedersen2018). On the other hand, studies conducted on patients with coeliac disease show that strict gluten restriction may lead to an unbalanced diet (eg. high sugar, high fat and low fibre) and create a high risk for nutritional deficiencies (eg. calcium and iron) raising concerns regarding potential effects of long-term adherence (Bardella et al., Reference Bardella, Fredella, Prampolini, Molteni, Giunta and Bianchi2000; Kinsey et al., Reference Kinsey, Burden and Bannerman2008; Wild et al., Reference Wild, Robins, Burley and Howdle2010). To the best of our knowledge, the nutritional challenges of a gluten-free diet have not yet been studied in IBS and its effect on the gut microenvironment is poorly understood. A gluten-free diet possibly improves bowel habits (Vazquez-Roque et al., Reference Vazquez-Roque, Camilleri, Smyrk, Murray, Marietta, O’Neill, Carlson, Lamsam, Janzow, Eckert, Burton and Zinsmeister2013) and modulates intestinal permeability (Vazquez-Roque et al., Reference Vazquez-Roque, Camilleri, Smyrk, Murray, Marietta, O’Neill, Carlson, Lamsam, Janzow, Eckert, Burton and Zinsmeister2013; Wu et al., Reference Wu, Vazquez-Roque, Carlson, Burton, Grover, Camilleri and Turner2017), and a diet low in both gluten and FODMAP has been suggested to improve IBS symptoms severity while normalising the gut microbiota composition in IBS patients (Naseri et al., Reference Naseri, Dabiri, Rostami-Nejad, Yadegar, Houri, Olfatifar, Sadeghi, Saadati, Ciacci, Iovino and Zali2021), but more studies are certainly needed on this topic in IBS (Table 4).
Current recommendations
To conclude, exclusion of certain food items seems to relieve symptoms in a large proportion of IBS patients, at least in the short term. Moreover, professional dietary guidance can prevent avoidance of food items crucial for health (McKenzie et al., Reference McKenzie, Bowyer, Leach, Gulia, Horobin, O’Sullivan, Pettitt, Reeves, Seamark, Williams, Thompson and Lomer2016) and improve quality of life and diet (Ostgaard et al., Reference Ostgaard, Hausken, Gundersen and El-Salhy2012). For the moment, the current guidelines carefully include the diet low in FODMAPs as a second-line dietary therapy for IBS management, because of its invasiveness, safety concerns and the low-quality evidence to support its long-term use to alleviate global IBS symptoms, even though the proof of its efficacy in the short term is good. Moreover, the low FODMAP diet is strict and requires guidance by a dietician. Due to the lack of understanding of long-term effects on nutritional adequacy and gut health, its implementation should be short-term (Moayyedi et al., Reference Moayyedi, Andrews, MacQueen, Korownyk, Marsiglio, Graff, Kvern, Lazarescu, Liu, Paterson, Sidani and Vanner2019; van Lanen et al., Reference van Lanen, de Bree and Greyling2021; Vasant et al., Reference Vasant, Paine, Black, Houghton, Everitt, Corsetti, Agrawal, Aziz, Farmer, Eugenicos, Moss-Morris, Yiannakou and Ford2021), and thereafter a gradual reintroduction of FODMAPs is recommended (Whelan et al., Reference Whelan, Martin, Staudacher and Lomer2018). The effect of a gluten-free diet, however, is unclear and there is consequently a recommendation against its widespread use in IBS. Dietary recommendations concerning other diets cannot be made due to lack of objective information. Food can be regarded as the crossroads between pathogenesis, symptom origin and symptom control (Spencer et al., Reference Spencer, Chey and Eswaran2014), and the importance of this intersection is expected to attract a lot of attention in the coming years.
Faecal microbiota transplantation
FMT is a 1,700-year-old, unconventional therapy (Zhang et al., Reference Zhang, Luo, Shi, Fan and Ji2012), which aims to favourably alter gut microbiota composition through administration of faecal material from healthy individuals into the GI tract of patients with presumed gut microbiota alterations. First applied to treat food poisoning and severe diarrhoea in the fourth century (Zhang et al., Reference Zhang, Luo, Shi, Fan and Ji2012) and now effectively used for treatment of Clostridium difficile infection, FMT has produced varying outcomes in IBS patients in regards to symptoms and gut microenvironment in research settings (König et al., Reference König, Siebenhaar, Högenauer, Arkkila, Nieuwdorp, Norén, Ponsioen, Rosien, Rossen, Satokari, Stallmach, de Vos, Keller and Brummer2017).
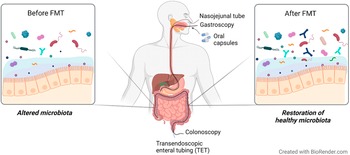
Several strategies are currently available to perform a FMT (Figure 3) and may explain the diversity of outcomes described in patients with IBS (Table 5). One way to administer FMT is through oral capsules, which have been shown to change the gut microbiota to resemble that of the donors. However, microbiota alterations do not seem to translate well into clinical improvement and contrary to what was expected, FMT treatment may have less or comparable efficacy to placebo (Aroniadis et al., Reference Aroniadis, Brandt, Oneto, Feuerstadt, Sherman, Wolkoff, Kassam, Sadovsky, Elliott, Budree, Kim and Keller2019; Halkjær et al., Reference Halkjær, Christensen, Lo, Browne, Günther, Hansen and Petersen2018). While this method is easy to administer and is cost effective, it may require the intake of multiple capsules a day, which can trigger feelings of nausea and vomiting (Gulati et al., Reference Gulati, Singh, Corrie, Kaur and Chandwani2020). Another approach is to deliver faecal material to the colon (Holster et al., Reference Holster, Lindqvist, Repsilber, Salonen, de Vos, König and Brummer2019; Lahtinen et al., Reference Lahtinen, Jalanka, Hartikainen, Mattila, Hillilä, Punkkinen, Koskenpato, Anttila, Tillonen, Satokari and Arkkila2020), commonly performed via colonoscopy (Gulati et al., Reference Gulati, Singh, Corrie, Kaur and Chandwani2020). This method of FMT may shift the recipient’s microbiota (Lahtinen et al., Reference Lahtinen, Jalanka, Hartikainen, Mattila, Hillilä, Punkkinen, Koskenpato, Anttila, Tillonen, Satokari and Arkkila2020), but does not seem to increase butyrate-producing bacteria following treatment (Holster et al., Reference Holster, Lindqvist, Repsilber, Salonen, de Vos, König and Brummer2019) and so far has not been demonstrated to be superior to the placebo group receiving autologous stool in improving symptom severity (Holster et al., Reference Holster, Lindqvist, Repsilber, Salonen, de Vos, König and Brummer2019; Lahtinen et al., Reference Lahtinen, Jalanka, Hartikainen, Mattila, Hillilä, Punkkinen, Koskenpato, Anttila, Tillonen, Satokari and Arkkila2020). Furthermore, the high cost and requirement of pre-treatment (ie. bowel cleansing), that may influence symptoms and gut microenvironment, make it difficult to interpret the clinical outcome of colonoscopic FMT (Gulati et al., Reference Gulati, Singh, Corrie, Kaur and Chandwani2020; Holster et al., Reference Holster, Lindqvist, Repsilber, Salonen, de Vos, König and Brummer2019). Alternatively, FMT delivered by transendoscopic enteral tubing (TET) does not require bowel preparation and is convenient for multiple administrations (Gulati et al., Reference Gulati, Singh, Corrie, Kaur and Chandwani2020). Delivery of microbiota isolates obtained from donor faeces using TET improved symptom severity and quality of life as well as changed the dominant microbial taxa in responders following FMT (Huang et al., Reference Huang, Chen, Luo, Xu, He, Li, Zhou, Yao, Nie and Zhou2019).

Figure 3 Faecal microbiota transplantation (FMT) as a strategy to restore healthy gut microbiota in IBS. Overall, FMT aims to shift the altered microbiota towards homeostasis through colonisation of healthy donor microbiota. The processed faecal material obtained from a healthy donor can be administered through upper (eg. oral capsules, gastroscopy and nasojejunal tube) or lower [eg. colonoscopy and transendoscopic enteral tubing (TET)] GI routes. Oral capsules generally contain very small amounts of faecal material and require multiple ingestions daily. FMT delivery through gastroscopy and nasojejunal route involves administration of a flexible tube through the mouth and nose, respectively, into the small intestine. In colonoscopy, FMT is delivered to the colon through a flexible tube inserted through the anus. The colonoscopy channel can also be used to introduce TET into the colon. The TET is then fixed to the mucosa using titanium clips for whole-colon FMT delivery.
Table 5. Overview of studies evaluating the effects of faecal microbiota transplantation on the gut microenvironment and clinical outcome in patients with IBS.
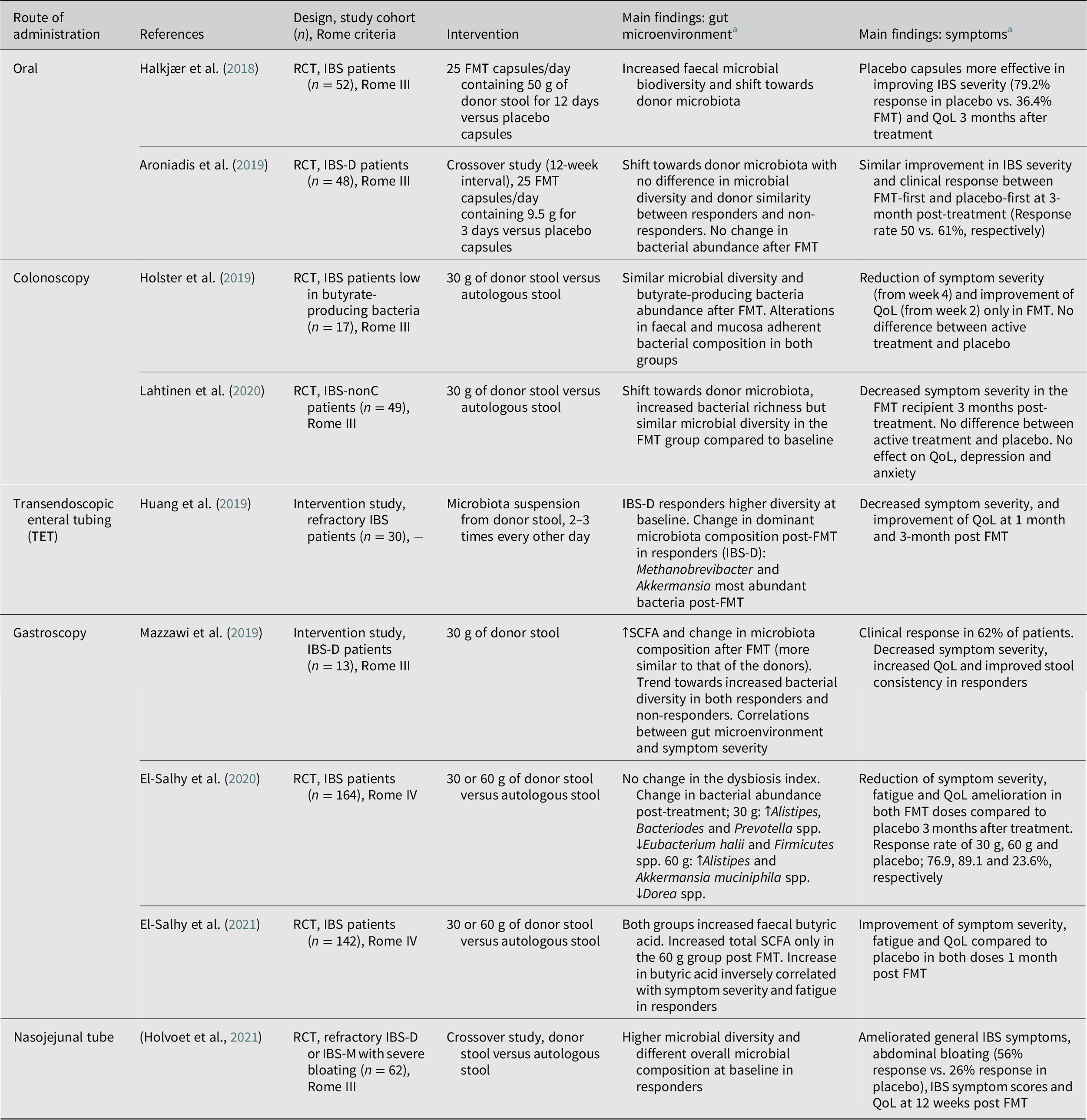
Abbreviations: FMT, faecal microbiota transplantation; IBS, irritable bowel syndrome; IBS-D, irritable bowel syndrome with diarrhoea; IBS-nonC, irritable bowel syndrome without constipation (= diarrhoea and mixed bowel habits); IBS-M, irritable bowel syndrome with mixed bowel habits; QoL, quality of life; RCT, randomised controlled trial; SCFA, short-chain fatty acid. Symbols: ↑, increase; ↓, decrease.
a Statistically significant findings unless otherwise specified.
Delivery of stool transplant through gastroscopy may normalise SCFA levels (El-Salhy et al., Reference El-Salhy, Valeur, Hausken and Gunnar Hatlebakk2021; Mazzawi et al., Reference Mazzawi, Hausken, Hov, Valeur, Sangnes, El-Salhy, Gilja, Hatlebakk and Lied2019) and change gut microbiota composition (Cruz-Aguliar et al., Reference Cruz-Aguliar, Wantia, Clavel, Vehreschild, Buch, Bajbouj, Haller, Busch, Schmid and Stein-Thoeringer2019; Mazzawi et al., Reference Mazzawi, Hausken, Hov, Valeur, Sangnes, El-Salhy, Gilja, Hatlebakk and Lied2019), while improving symptoms and QoL in IBS patients (Cruz-Aguliar et al., Reference Cruz-Aguliar, Wantia, Clavel, Vehreschild, Buch, Bajbouj, Haller, Busch, Schmid and Stein-Thoeringer2019; El-Salhy et al., Reference El-Salhy, Hatlebakk, Gilja, Bråthen Kristoffersen and Hausken2020; Mazzawi et al., Reference Mazzawi, Hausken, Hov, Valeur, Sangnes, El-Salhy, Gilja, Hatlebakk and Lied2019). Some of the changes in the gut microenvironment correlated with IBS symptom severity in the patients responding to the FMT treatment (Cruz-Aguliar et al., Reference Cruz-Aguliar, Wantia, Clavel, Vehreschild, Buch, Bajbouj, Haller, Busch, Schmid and Stein-Thoeringer2019; El-Salhy et al., Reference El-Salhy, Valeur, Hausken and Gunnar Hatlebakk2021). Similar to gastroscopy, the use of nasojejunal tube to deliver FMT has led to promising results including improvements in general IBS symptoms, abdominal bloating, quality of life, and decrease in selected symptom scores in the active treatment group compared to the placebo group receiving autologous stool (Holvoet et al., Reference Holvoet, Joossens, Vázquez-Castellanos, Christiaens, Heyerick, Boelens, Verhasselt, van Vlierberghe, De Vos, Raes and De Looze2021). Furthermore, responders had higher microbial diversity and different overall microbial composition at baseline compared with non-responders (Holvoet et al., Reference Holvoet, Joossens, Vázquez-Castellanos, Christiaens, Heyerick, Boelens, Verhasselt, van Vlierberghe, De Vos, Raes and De Looze2021). Although costly, FMT delivery through gastroscopy and nasojejunal tube, are potentially less invasive than colonoscopy and can be performed without bowel cleansing (Gulati et al., Reference Gulati, Singh, Corrie, Kaur and Chandwani2020).
Current recommendations
Considering the relatively benign nature of IBS, and the potential risks with FMT, the general concern has been whether it is worth the trade-off. While there is a call for caution (Camilleri, Reference Camilleri2021), the adverse effects of FMT in IBS patients have been limited to short-term and self-limiting GI symptoms. As of now, FMT is not an accepted therapy for clinical use in IBS and should still be limited to clinical trials (König et al., Reference König, Siebenhaar, Högenauer, Arkkila, Nieuwdorp, Norén, Ponsioen, Rosien, Rossen, Satokari, Stallmach, de Vos, Keller and Brummer2017), even though recently published large controlled FMT trials provide hope for future use in selected patients (El-Salhy et al., Reference El-Salhy, Hatlebakk, Gilja, Bråthen Kristoffersen and Hausken2020; Holvoet et al., Reference Holvoet, Joossens, Vázquez-Castellanos, Christiaens, Heyerick, Boelens, Verhasselt, van Vlierberghe, De Vos, Raes and De Looze2021). Still, the efficacy is debatable, which could be attributed to differences in the methods used (Table 5). Overall, FMT delivery through gastroscopy or via a nasojejunal tube have been the most promising options and showed dose-dependent and long-term improvements in IBS patients (El-Salhy et al., Reference El-Salhy, Hatlebakk, Gilja, Bråthen Kristoffersen and Hausken2020, Reference El-Salhy, Kristoffersen, Valeur, Casen, Hatlebakk, Gilja and Hausken2022; Holvoet et al., Reference Holvoet, Joossens, Vázquez-Castellanos, Christiaens, Heyerick, Boelens, Verhasselt, van Vlierberghe, De Vos, Raes and De Looze2021). The positive outcome can be attributed to the identification of a donor with well-defined favourable microbiota (diverse and stable over time) and recipients that are more likely to respond to the treatment (diverse and less disturbed microbiota) (El-Salhy et al., Reference El-Salhy, Hatlebakk, Gilja, Bråthen Kristoffersen and Hausken2020; Holvoet et al., Reference Holvoet, Joossens, Vázquez-Castellanos, Christiaens, Heyerick, Boelens, Verhasselt, van Vlierberghe, De Vos, Raes and De Looze2021). Overall, while these results are encouraging, data regarding the optimal FMT protocol (eg. delivery route, dose and frequency) and mechanisms underlying symptom improvement are yet to be established.
Combined treatments
The treatment strategies described in this review have yielded some clinical benefits, but with mixed and sometimes inconsistent results regarding efficacy. To overcome the limitations of single treatment interventions, combinations of different strategies have been tested.
Restrictive dietary interventions, while improving symptoms, may paradoxically have adverse effects on the gut microenvironment. The low FODMAP diet, a well-recognised IBS therapy, has been shown to reduce faecal bifidobacterial abundances and butyrate as well as total SCFA levels, which otherwise are associated with health benefit (Huaman et al., Reference Huaman, Mego, Manichanh, Cañellas, Cañueto, Segurola, Jansana, Malagelada, Accarino, Vulevic, Tzortzis, Gibson, Saperas, Guarner and Azpiroz2018; Staudacher et al., Reference Staudacher, Lomer, Farquharson, Louis, Fava, Franciosi, Scholz, Tuohy, Lindsay, Irving and Whelan2017; Wilson et al., Reference Wilson, Rossi, Kanno, Parkes, Anderson, Mason, Irving, Lomer and Whelan2020). Conversely, prebiotics have a bifidogenic effect and have been suggested as a supplementary treatment for the low FODMAP diet to potentially overcome the negative effects on gut microbiota composition and function with this restrictive diet (Huaman et al., Reference Huaman, Mego, Manichanh, Cañellas, Cañueto, Segurola, Jansana, Malagelada, Accarino, Vulevic, Tzortzis, Gibson, Saperas, Guarner and Azpiroz2018). Interestingly, co-administration of a probiotic mixture containing Bifidobacterium spp., together with a low FODMAP diet, increased faecal Bifidobacterium and Lactobacillus abundances (Staudacher et al., Reference Staudacher, Lomer, Farquharson, Louis, Fava, Franciosi, Scholz, Tuohy, Lindsay, Irving and Whelan2017, Reference Staudacher, Scholz, Lomer, Ralph, Irving, Lindsay, Fava, Tuohy and Whelan2021). However, supplementation of β-GOS during the low FODMAP diet improved symptoms, but did not lead to increase in bifidobacteria (Wilson et al., Reference Wilson, Rossi, Kanno, Parkes, Anderson, Mason, Irving, Lomer and Whelan2020). On the other hand, high levels of FOS (16 g/day), while increasing bifidobacteria, led to worsening of symptoms in IBS patients on a low FODMAP diet (Hustoft et al., Reference Hustoft, Hausken, Ystad, Valeur, Brokstad, Hatlebakk and Lied2017), highlighting the importance of the dose used.
Another strategy for combined therapy is to co-administer probiotics together with prebiotics, now referred to as complementary synbiotics. Synbiotics are “a mixture comprising live microorganisms and substrate(s) selectively utilised by host microorganisms that confers a health benefit on the host” (Swanson et al., Reference Swanson, Gibson, Hutkins, Reimer, Reid, Verbeke, Scott, Holscher, Azad, Delzenne and Sanders2020). According to this definition, synbiotics may also comprise live organisms and substrates that are not probiotics and prebiotics respectively, which in this case are referred to as synergistic synbiotics (Swanson et al., Reference Swanson, Gibson, Hutkins, Reimer, Reid, Verbeke, Scott, Holscher, Azad, Delzenne and Sanders2020). The delivery of synbiotic fermented milk, which included inulin (90 per cent) and oligofructose (10 per cent) as prebiotics together with the probiotics Lactobacillus and bifidobacteria, demonstrated transient increase in administered strains in faecal samples of IBS patients (Bogovič Matijašić et al., Reference Bogovič Matijašić, Obermajer, Lipoglavšek, Sernel, Locatelli, Kos, Šmid and Rogelj2016). However, the effects of synbiotics on IBS symptoms vary across studies (Chlebicz-Wójcik and Śliżewska, Reference Chlebicz-Wójcik and Śliżewska2021).
Metabolites
Metabolites produced or modulated by intestinal bacteria have been suggested to play a role in IBS pathophysiology, either directly by chemical interactions or via local modulation of microbiota and/or immune activity (Rajilić-Stojanović et al., Reference Rajilić-Stojanović, Jonkers, Salonen, Hanevik, Raes, Jalanka, de Vos, Manichanh, Golic, Enck, Philippou, Iraqi, Clarke, Spiller and Penders2015). SCFAs and bile acids are two of the most targeted metabolites in IBS related research and regarded as potential treatment targets.
Short-chain fatty acids
SCFAs, mainly acetate, propionate, and butyrate, are the most prominent by-products of colonic microbiota-mediated metabolism of indigestible dietary fibre. Their established role in intestinal homeostasis (Tan et al., Reference Tan, McKenzie, Potamitis, Thorburn, Mackay and Macia2014) and the recent suggestion of their involvement in microbiota-gut–brain interaction (Dalile et al., Reference Dalile, Van Oudenhove, Vervliet and Verbeke2019) have attracted interest to study SCFAs in IBS pathophysiology. Some IBS patients may have lower levels of SCFA-producing bacteria (Pozuelo et al., Reference Pozuelo, Panda, Santiago, Mendez, Accarino, Santos, Guarner, Azpiroz and Manichanh2015). Furthermore, a recent meta-analysis indicates globally lower SCFA levels, including butyrate, in IBS-C patients and higher SCFA levels in IBS-D patients (Sun et al., Reference Sun, Jia, Song and Duan2019). In line with this, it has been demonstrated that receiving microencapsulated sodium butyrate for 12 weeks, as a supplemental therapy, reduced the frequency of spontaneous and postprandial abdominal pain, the pain during defecation and constipation compared to placebo, while there were no significant effects on the severity of other GI symptoms (Banasiewicz et al., Reference Banasiewicz, Krokowicz, Stojcev, Kaczmarek, Kaczmarek, Maik, Marciniak, Krokowicz, Walkowiak and Drews2013). Furthermore, modulation of SCFAs, described in other gut microbiota-targeted therapies along with association of SCFAs to health-promoting bacteria, may serve as a potential marker linked to symptom improvement (see Tables 1–5).
Bile acid metabolism
Bile acids are synthesised in the liver and released into the intestinal lumen to be mostly reabsorbed upon reaching the terminal ileum, while the remaining bile acids (<5 per cent) pass into the colon. Once in the colon, primary bile acids are modified by the bacteria via enzymatic processes, releasing secondary bile acids that mediate series of signalling events (Zhan et al., Reference Zhan, Zheng, Li, Wu, Qin, Luo and Huang2020). Approximately one third of the IBS-D patients, are reported to have bile acid malabsorption (BAM) (Wedlake et al., Reference Wedlake, A’Hern, Russell, Thomas, Walters and Andreyev2009), which leads to increased amounts of bile acids reaching the colon, where they can stimulate intestinal motility and secretion and potentially also exaggerate visceral hypersensitivity (Bajor et al., Reference Bajor, Törnblom, Rudling, Ung and Simrén2015; Dior et al., Reference Dior, Delagrèverie, Duboc, Jouet, Coffin, Brot, Humbert, Trugnan, Seksik, Sokol, Rainteau and Sabate2016; Wei et al., Reference Wei, Wang, Zhang, Zhang, Niu and Yao2020). Bile acid sequestrants are part of the recommended therapy for diarrhoea in IBS patients despite limited supporting evidence from large randomised controlled trials. However, side effects and possible interference with other medications may make long-term use problematic (Nee et al., Reference Nee, Zakari and Lembo2015). Several bacteria involved in bile acid metabolism seem to be altered in IBS patients (Zhan et al., Reference Zhan, Zheng, Li, Wu, Qin, Luo and Huang2020). Potentially Clostridia species, enriched in a subset of IBS-D patients, suppress the feedback-loop regulating bile acid synthesis through metabolites, further increasing colonic bile acid exposure (Zhao et al., Reference Zhao, Yang, Chen, Huang, Lu, Lin, Huang, Ning, Zhai, Zhong, Lam, Yang, Zhang, Cheng, Han, Qiu, Shang, Huang, Xiao, Ren, Chen, Sun, El-Nezami, Cai, Lu, Fang, Jia and Bian2020). Accordingly, as shown by a longitudinal study, the microbial biotransformation of bile acids is altered in both IBS-C and IBS-D patients, with IBS-D patients having higher and IBS-C patients having lower faecal primary bile acid levels compared to healthy individuals. Furthermore, the primary bile acids were increased in both IBS groups during flares (Mars et al., Reference Mars, Yang, Ward, Houtti, Priya, Lekatz, Tang, Sun, Kalari, Korem, Bhattarai, Zheng, Bar, Frost, Johnson, van Treuren, Han, Ordog, Grover, Sonnenburg, D’Amato, Camilleri, Elinav, Segal, Blekhman, Farrugia, Swann, Knights and Kashyap2020). While changes in gut microbiota composition seem to play an important role in altered bile acid metabolism, the microbiota-bile acid axis is rarely assessed in studies on antibiotic, dietary, prebiotic and probiotic interventions. Indeed, the microbiota-bile acid interaction may be involved in explaining the effects of the aforementioned interventions on symptoms and could be a valid treatment target in future IBS trials.
Current recommendations
Abnormalities in metabolite profiles may partially reflect affected biological pathways involved in IBS symptom generation, further reflected in metabolite alterations specific to each IBS subtype. Targeting the gut microenvironment through intervention studies (eg. antibiotics, probiotics, prebiotics and FMT), with thorough analysis of secondary effects on metabolites may be important for more effective long-term therapeutic strategies. Furthermore, metabolomic alterations, may also provide mechanistic insights into treatment response, as suggested by a post hoc analysis of a dietary intervention study (Nybacka et al., Reference Nybacka, Simrén, Störsrud, Törnblom, Winkvist and Lindqvist2021). Overall, while the role of SCFA is extensively studied, the role of other metabolites, such as bile acids, neurotransmitters and vitamins, remains to be explored (James et al., Reference James, Fraser, Young, McNabb and Roy2020).
Conclusion
IBS is a multifaceted disorder with a complex pathophysiology, where the gut microenvironment seems to play a key role in symptom generation. Evidence suggest that multiple luminal factors may be associated with IBS, including gut microbiota, microbial and dietary metabolites, and immune-related factors. Therefore, interventions targeting the gut microenvironment may be a coherent approach to indirectly provide health-related benefit to patients with IBS. This narrative review gathers the current knowledge on strategies that consider gut microenvironment modulation as a therapeutic target, with a specific focus on the link between effects in the gut microenvironment and impact on IBS symptoms.
Antibiotics, probiotics, prebiotics, food and FMT are strategies with potential to manage IBS symptoms (Figure 1). They vary in their effect on the gut microenvironment and present some limitations. For antibiotics, we largely lack mechanistic understanding on symptom improvement, especially regarding their effect on microbiota and how relevant this is for symptom improvement. The effect on the gut microenvironment may be highly dependent on dose and/or type of probiotics and prebiotics, and the link to symptom improvement also remains to be fully established. Certain dietary interventions seem to have favourable effects on symptoms, but long-term commitment may lead to undesired effects on the gut microenvironment, for example, a strict low-FODMAP diet reduces health-associated bacteria in the gut. The heterogeneity in study designs in FMT makes it difficult to draw conclusions on efficacy and the impact on the gut microenvironment, as well as mechanisms behind symptom improvement and factors of importance for donor selection. Still, combination of certain therapies might be an alternative approach, although solid scientific support for this approach is needed before being implemented in the clinic. In addition, a better understanding of the importance of metabolomic alterations reflecting host and microbial metabolism could help in developing more evidence-based therapies and predicting potential response in the individual patient.
To conclude, treatments targeting the gut microenvironment are promising, but improved knowledge regarding their specific mode of action, mechanisms underlying their effects on symptoms, the effect of different doses, is still needed to potentially be able to identify the right treatment to the right patient.
Disclosure statement
C.I. and L.M. have no conflict of interest to disclose. M.S. has received unrestricted research grants from Danone Nutricia Research and Glycom A/S (now DSM), and served as a Consultant/Advisory Board member for Danone Nutricia Research, Ironwood, Menarini, Biocodex, Genetic Analysis AS, Glycom A/S (now DSM), Tillotts, Arena and Adnovate, and as in the speakers’ bureau for Tillotts, Menarini, Kyowa Kirin, Takeda, Shire, Biocodex, Alimentary Health, AlfaSigma and Falk Foundation. L.Ö. has received financial support for research from Genetic Analysis AS, Biocodex, Danone Research and AstraZeneca and served as Consultant/Advisory Board member for Genetic Analysis AS, and as a speaker for Biocodex, Ferring Pharmaceuticals, Takeda, AbbVie and Meda.
Notes on contributors
C.I. has recently defended her PhD thesis at Sahlgrenska Academy, University of Gothenburg. Her research focuses on the host-microbiota crosstalk and the effect of the prebiotic human milk oligosaccharides in patients with irritable bowel syndrome (IBS). L.M. is a PhD student in the Sahlgrenska Academy, University of Gothenburg. Her research focuses on microbiologic and immunologic factors behind non-classical food allergy in patients with IBS. L.Ö is a professor of Immunology at Sahlgrenska Academy, University of Gothenburg. She has numerous publications focusing on describing gut microbiota, immune profile and the link to disease profile, as well as therapy outcome in patients with inflammatory bowel disease and patients with IBS. M.S. is a professor of Gastroenterology at Sahlgrenska Academy, University of Gothenburg; Senior Consultant at Sahlgrenska University Hospital and Adjunct Professor of Medicine at the University of North Carolina School of Medicine. His research focuses on the pathogenesis and pathophysiology of functional gastrointestinal disorders and their treatments.
Supplementary Materials
To view supplementary material for this article, please visit http://doi.org/10.1017/gmb.2022.6.
Author contributions
Conceptualization: M.S.; Supervision: L.Ö., M.S.; Visualisation: C.I., L.M.; Writing – original draft: C.I., L.M.; Writing – review and editing: C.I., L.M., L.Ö., M.S. All authors have read and approved the final version of this manuscript.
Funding
The study was funded by Swedish Medical Research Council [grant nos. 2019-01052 (L.Ö.) and 2018-02566 (M.S.)], Sahlgrenska Academy at University of Gothenburg (L.Ö.), Faculty of Medicine at University of Gothenburg (M.S.), grants from the Swedish state under the agreement between the Swedish government and the county councils; the ALF-agreement, ALFGBG-723921 (L.Ö.), ALFGBG-722331 (M.S.) and Regional Executive Board, Region Västra Götaland [grant no. VGFOUREG-931919 (L.Ö.)].