1. Introduction
The end-Triassic mass extinction (201.36±0.17 Ma; Schoene et al. Reference Schoene, Guex, Bartolini, Schaltegger and Blackburn2010; Wotzlaw et al. Reference Wotzlaw, Guex, Bartolini, Gallet, Krystyn, McRoberts, Taylor, Schoene and Schaltegger2014) is considered as one of the five largest Phanerozoic extinction events (Raup & Sepkoski, Reference Raup and Sepkoski1982; Sepkoski, Reference Sepkoski and Walliser1996; Hesselbo, McRoberts & Pálfy, Reference Hesselbo, McRoberts and Pálfy2007), and massive biotic crises occurred in both the marine and terrestrial realms (Colbert, Reference Colbert1958; Pálfy et al. Reference Pálfy, Mortensen, Carter, Smith, Friedman and Tipper2000; Hallam, Reference Hallam2002; Hesselbo et al. Reference Hesselbo, Robinson, Surlyk and Piasecki2002; Olsen et al. Reference Olsen, Kent, Sues, Koeberl, Huber, Montanari, Rainforth, Fowell, Szajna and Hartline2002; Akikuni et al. Reference Akikuni, Hori, Vajda, Grant Mackie and Ikehara2010). In the ocean, the conodont animal became extinct and corals and molluscs such as bivalves and ammonites were seriously affected (Hallam, Reference Hallam, Sharpton and Ward1990, Reference Hallam2002; Tanner, Lucas & Chapman, Reference Tanner, Lucas and Chapman2004; Lucas & Tanner, Reference Lucas and Tanner2007, Reference Lucas, Tanner and Elewa2008; Lathuilière & Marchal, Reference Lathuilière and Marchal2009); on land, amphibians and reptiles suffered major losses (Colbert, Reference Colbert1958; Olsen, Shubin & Anders, Reference Olsen, Shubin and Anders1987; Milner, Reference Milner1989; Benton, Reference Benton1991; Tanner, Lucas & Chapman, Reference Tanner, Lucas and Chapman2004; Lucas & Tanner, Reference Lucas, Tanner and Elewa2008). Within plant ecosystems, a major change took place with both reorganization and extinctions (McElwain et al. Reference McElwain, Popa, Hesselbo, Haworth and Surlyk2007; McElwain, Wagner & Hesselbo, Reference McElwain, Wagner and Hesselbo2009; Wang et al. Reference Wang, Fu, Xie, Huang, Li, Li, Liu, Yu, Pan, Tian and Jiang2010; Vajda & Bercovici, Reference Vajda and Bercovici2014; Sha et al. Reference Sha, Olsen, Pan, Xu, Wang, Zhang, Yao and Vajda2015; Lindström, Reference Lindström2016). Widespread magmatic activity of the Central Atlantic Magmatic Province (CAMP) has repeatedly been invoked to have triggered this catastrophic event (Marzoli et al. Reference Marzoli, Renne, Piccirillo, Ernesto, Bellieni and De Min1999, Reference Marzoli, Bertrand, Knight, Cirilli, Buratti, Vérati, Nomade, Renne, Youbi, Martini, Allenbach, Neuwerth, Rapaille, Zaninetti and Bellieni2004; Wignall, Reference Wignall2001; Hesselbo et al. Reference Hesselbo, Robinson, Surlyk and Piasecki2002; Hesselbo, McRoberts & Pálfy, Reference Hesselbo, McRoberts and Pálfy2007; van de Schootbrugge & Wignall, Reference van de Schootbrugge and Wignall2016). The most commonly accepted killing mechanism is rapid global warming driven by outgassing of CO2 and release of methane (McElwain, Beerling & Woodward, Reference McElwain, Beerling and Woodward1999; Tanner, Lucas & Chapman, Reference Tanner, Lucas and Chapman2004; Bonis, Ruhl & Kürschner, Reference Bonis, Ruhl and Kürschner2010; Whiteside et al. Reference Whiteside, Olsen, Eglinton, Brookfield and Sambrotto2010; Ruhl et al. Reference Ruhl, Bonis, Reichart, Damsté and Küerschner2011; Schaller, Wright & Kent, Reference Schaller, Wright and Kent2011; Steinthorsdottir, Jeram & McElwain, Reference Steinthorsdottir, Jeram and McElwain2011; Schaller et al. Reference Schaller, Wright, Kent and Olsen2012), and acidification of surface waters and terrestrial environments (van de Schootbrugge et al. Reference van de Schootbrugge, Quan, Lindström, Püttmann, Heunisch, Pross, Fiebig, Petschick, Röhling, Richoz, Rosenthal and Falkowski2009; Greene et al. Reference Greene, Martindale, Ritterbush, Bottjer, Corsetti and Berelson2012; Hönisch et al. Reference Hönisch, Ridgwell, Schmidt, Thomas, Gibbs, Sluijs, Zeebe, Kump, Martindale, Greene, Kiessling, Ries, Zachos, Royer, Barker, Marchitto, Moyer, Pelejero, Ziveri, Foster and Williams2012; Richoz et al. Reference Richoz, van de Schootbrugge, Pross, Püttmann, Quan, Lindström, Heunisch, Fiebig, Maquil, Schouten, Hauzenberger and Wignall2012; Callegaro et al. Reference Callegaro, Baker, De Min, Marzoli, Geraki, Bertrand, Viti and Nestola2014; Ikeda et al. Reference Ikeda, Hori, Okada and Nakada2015; Bachan & Payne, Reference Bachan and Payne2016; van de Schootbrugge & Wignall, Reference van de Schootbrugge and Wignall2016).
In the palaeobotanical record, the end-Triassic event is typified by extinction of seed ferns including Lepidopteris, and the void was soon taken by dipteridacean ferns such as Thaumatopteris and a flora rich in conifers, ginkgoaleans and bennettites (McElwain et al. Reference McElwain, Popa, Hesselbo, Haworth and Surlyk2007; Pott & McLoughlin, Reference Pott and McLoughlin2009, Reference Pott and McLoughlin2011; Vajda, Calner & Ahlberg, Reference Vajda, Calner and Ahlberg2013; Mander, Kürschner & McElwain, Reference Mander, Kürschner and McElwain2013). This dramatic change is also expressed in the palynological record where Rhaetian and, in some places, early Hettangian successions host abnormal abundances of the enigmatic gymnosperm pollen Ricciisporites tuberculatus (Bonis, Ruhl & Kürschner, Reference Bonis, Ruhl and Kürschner2010; Mander, Kürschner & McElwain, Reference Mander, Kürschner and McElwain2013; Vajda, Calner & Ahlberg, Reference Vajda, Calner and Ahlberg2013; Lindström, Reference Lindström2016). In the European record, a transitional zone dominated by fern spores has been identified (Ruckwied et al. Reference Ruckwied, Götz, Pálfy and Torok2008; Götz et al. Reference Götz, Ruckwied, Pálfy and Haas2009; Larsson, Reference Larsson2009; Ruckwied & Götz, Reference Ruckwied and Götz2009; van de Schootbrugge et al. Reference van de Schootbrugge, Quan, Lindström, Püttmann, Heunisch, Pross, Fiebig, Petschick, Röhling, Richoz, Rosenthal and Falkowski2009; Pieńkowski, Niedźwiedzki & Waksmundzka, Reference Pieńkowski, Niedźwiedzki and Waksmundzka2012; Vajda, Calner & Ahlberg, Reference Vajda, Calner and Ahlberg2013; Lindström, Reference Lindström2016). This interval in the Swedish record has been formalized as the ‘Transitional Spore Spike Interval’ (TSI) by Larsson (Reference Larsson2009). This interval of pioneer vegetation is followed by the Hettangian floras characterized by high portions of Classopollis (Cheirolepidiaceae) recorded, for example from Sweden (Lund, Reference Lund1977; Guy-Ohlson, Reference Guy-Ohlson1981; Vajda, Calner & Ahlberg, Reference Vajda, Calner and Ahlberg2013), Greenland (Pedersen & Lund, Reference Pedersen and Lund1980; Mander, Kürschner & McElwain, Reference Mander, Kürschner and McElwain2013) and elsewhere.
With regards to the duration of the end-Triassic extinction, many workers argue that the extinction occurred over a prolonged interval marked by a series of discrete extinction events during Carnian–Rhaetian, rather than a single mass extinction at the end of the Rhaetian Age (Benton, Reference Benton1986; Hallam, Reference Hallam2002; Tanner, Lucas & Chapman, Reference Tanner, Lucas and Chapman2004; Bambach, Reference Bambach2006; Lucas & Tanner, Reference Lucas, Tanner and Elewa2008, Reference Lucas and Tanner2015; Wignall & van de Schootbrugge, Reference Wignall and van de Schootbrugge2016); studies on the Late Triassic ecosystem therefore form a very important role for better understanding the environmental changes prior to the end-Triassic extinction event.
In East Asia, the end-Triassic palaeobotanical and palynological records are somewhat scarce compared with Europe and North America. Two prime localities have so far been identified in China, both with well-exposed successions spanning the Triassic–Jurassic boundary, yielding diverse mega- and micro-floral records from terrestrial ecosystems. The regions include the Junggar Basin in the northwestern part of the country (Deng et al. Reference Deng, Lu, Fan, Pan, Cheng, Fu, Wang, Pan, Shen, Wang, Zhang, Jia, Duan and Fang2010; Sha et al. Reference Sha, Vajda, Pan, Larsson, Yao, Zhang, Wang, Cheng, Jiang, Deng, Chen and Peng2011, Reference Sha, Olsen, Pan, Xu, Wang, Zhang, Yao and Vajda2015) and the Sichuan Basin in southwestern China (Wang et al. Reference Wang, Fu, Xie, Huang, Li, Li, Liu, Yu, Pan, Tian and Jiang2010). In particular, the Upper Triassic strata of the Xujiahe Formation are well developed in the Sichuan Basin, and contain diverse fossil plant assemblages (Ye et al. Reference Ye, Liu, Huang, Chen, Peng, Xu and Zhang1986; Huang & Lu, Reference Huang and Lu1992; Huang, Reference Huang1995; Wang et al. Reference Wang, Fu, Xie, Huang, Li, Li, Liu, Yu, Pan, Tian and Jiang2010). Recent magnetostratigraphic studies revealed that the age of the Xujiahe Formation ranges from latest Norian to Rhaetian (from 207.2 Ma to 201.3 Ma at Qilixia, Xuanhan, northeastern Sichuan Basin) (Li et al. Reference Li, Zhang, Huang, Ogg, Hinnov, Wang, Zou and Li2017). Previous palynological studies on the Upper Triassic successions within the Sichuan Basin have contributed much to understanding the diversity and stratigraphy of the basin (Li, Reference Li1974; Cao & Huang, Reference Cao and Huang1980; Liu, Reference Liu1982; Bai et al. Reference Bai, Lu, Chen and Long1983; Zhang, Reference Zhang1984; Lu & Wang, Reference Lu and Wang1987; Yuan, Reference Yuan1989; Huang, Reference Huang1991; Shang & Li, Reference Shang and Li1992; Wang et al. Reference Wang, Kan, Liu, Liang and Zhu2008, Reference Wang, Fu, Xie, Huang, Li, Li, Liu, Yu, Pan, Tian and Jiang2010; Li & Wang, Reference Li and Wang2016). Recent efforts to decipher the regional responses of terrestrial plant communities prior to the end-Triassic event have been undertaken at several localities within the Sichuan Basin (Liu, Li & Wang, Reference Liu, Li and Wang2015a, Reference Liu, Li and Wangb; Li et al. Reference Li, Wang, Liu, Zhou and Wang2016). However, studies on the Late Triassic terrestrial palaeoenvironment based on palynology are still poorly documented in this region, thus our understanding on the Late Triassic ecosystem in the Sichuan basin therefore needs to be enhanced.
The present study documents a detailed palynological record from the Upper Triassic Xujiahe Formation of the Tanba section in the Hechuan region, southern Sichuan Basin (Fig. 1). Based on our palynological data, we aim to: (1) describe the Late Triassic vegetation in terms of abundance and diversity; and (2) decipher the Late Triassic climate variations in the studied area and place the results in a broader palaeogeographical context of the Late Triassic Period.
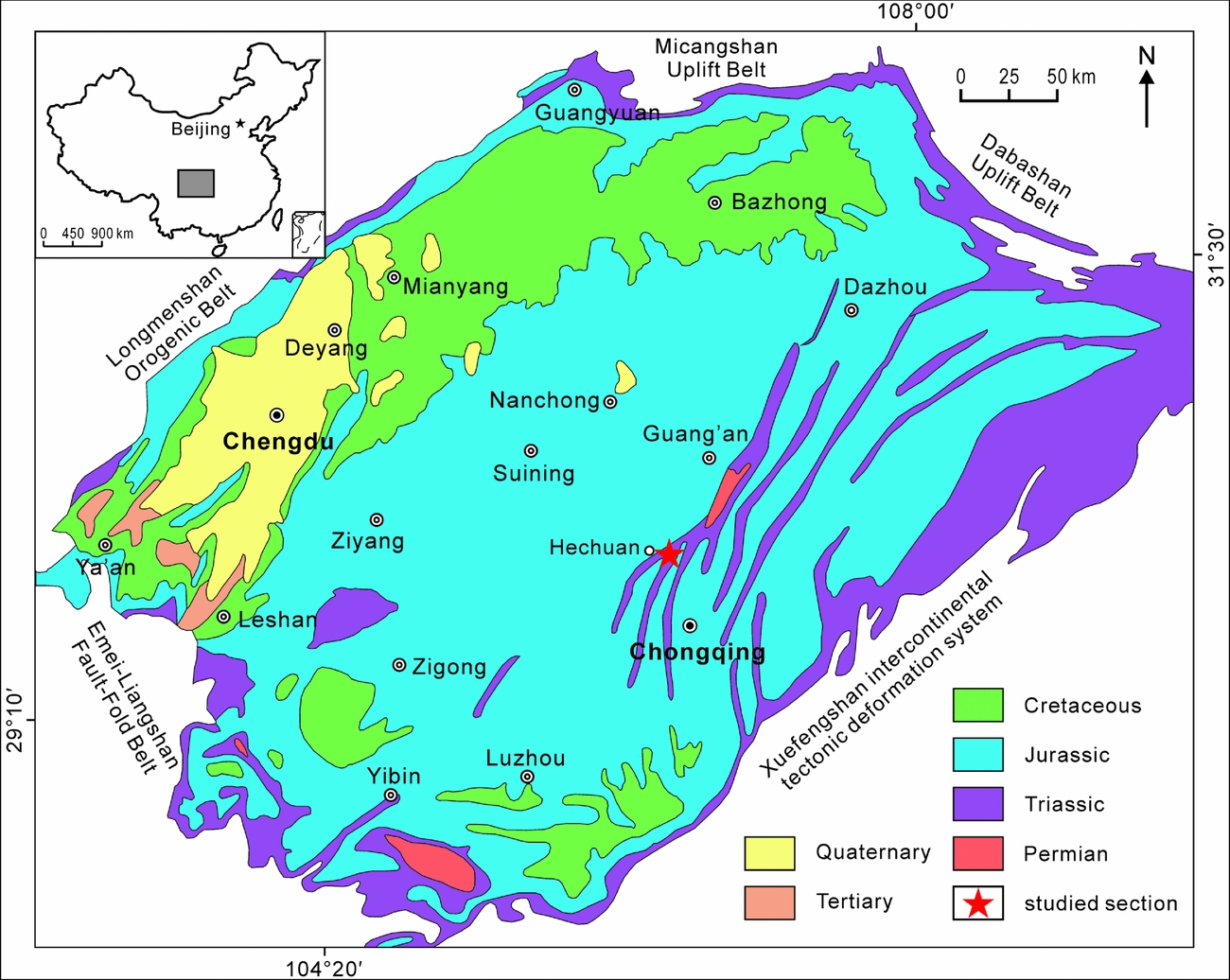
Figure 1. Simplified geological map of the Sichuan Basin showing the geological background and the location of the studied section (modified from Wang et al. Reference Wang, Fu, Xie, Huang, Li, Li, Liu, Yu, Pan, Tian and Jiang2010).
2. Geological setting
Located at the western margin of the South China block and the eastern margin of the Tibet Plateau, the Sichuan Basin is a large terrestrial petroliferous and coal-bearing basin, covering an area of 260000 km2 (Wang et al. Reference Wang, Fu, Xie, Huang, Li, Li, Liu, Yu, Pan, Tian and Jiang2010). It is bounded to the west by the Longmenshan orogenic belt, to the east by the Xuefengshan intercontinental tectonic deformation system, to the north by the Micangshan and Dabashan uplift belts, and to the south by the Emeishan–Liangshan fault-fold belt (Wang et al. Reference Wang, Fu, Xie, Huang, Li, Li, Liu, Yu, Pan, Tian and Jiang2010). Palaeozoic – early Mesozoic marine strata are well developed in the adjacent mountain areas, including Precambrian, Cambrian, Ordovician, Silurian, Carboniferous, Permian and Lower–Middle Triassic deposits. The Upper Triassic is dominated by terrestrial successions, mainly distributed in the eastern and northeastern margin of the basin. The remaining part of the basin is covered by massive Jurassic and Cretaceous red beds (Wang et al. Reference Wang, Fu, Xie, Huang, Li, Li, Liu, Yu, Pan, Tian and Jiang2010; Fig. 1).
Most importantly, the Upper Triassic strata represented by the Xujiahe Formation mainly consist of coal-bearing clastic rocks deposited in an inland lacustrine–fluvial–coal-swamp environment, varying over 400–650 m in thickness (Wang et al. Reference Wang, Fu, Xie, Huang, Li, Li, Liu, Yu, Pan, Tian and Jiang2010). The coal seams, which contain diverse plant remains (e.g. Xujiahe flora), play an important economic and scientific role in the Sichuan Basin (Wang et al. Reference Wang, Fu, Xie, Huang, Li, Li, Liu, Yu, Pan, Tian and Jiang2010).
The Xujiahe Formation is well exposed at the Tanba Section in the Hechuan region, southern Sichuan Basin (Fig. 1), administratively belonging to Chongqing City. The Xujiahe Formation overlies the Middle Triassic marine Leikoupo Formation and is, in turn, conformably overlain by the terrestrial Lower Jurassic Zhenzhuchong Formation (Fig. 2). At the Tanba section, an c. 500m outcrop of the Xujiahe Formation is well exposed. The lithology mainly comprises sandstones, siltstones, mudstones and coal beds, yielding a diverse and rich fossil assemblages of plants and bivalves. The Xujiahe Formation is subdivided into six lithological members (I–VI), numbered in ascending order. Members I, III and V are mainly dominated by mudstones and thin coal beds, representing floodplain–lacustrine and coal swamp deposits, whereas members II, IV and VI mainly comprise sandstones, representing fluvial-delta deposits (Fu et al. Reference Fu, Zhang, Yuan and Chen2010; Wang et al. Reference Wang, Fu, Xie, Huang, Li, Li, Liu, Yu, Pan, Tian and Jiang2010; Fig. 2).

Figure 2. Stratigraphic column of the Tanba Section of the Xujiahe Formation, indicating the beds sampled for palynology.
3. Materials and methods
Thirty-three palynological samples were collected from the Upper Triassic Xujiahe Formation (from the members I–VI) across the Tanba section in the Hechuan region. Eighteen samples were productive and yielded well-preserved and rich miospores (spores and pollen). No productive samples were however recovered from Member II (Fig. 2). All the productive samples were collected from organic-rich mudstones, siltstone and coal, therefore minimizing the taphonomic bias.
For palynological preparation, approximately 30 g of sediment was treated with HCl and HF to remove carbonates and silicate minerals, respectively. The residue of each sample was then washed with distilled water until a neutral pH was reached. The residue was subsequently sieved through a 10 μm size mesh. Finally, the palynomorph-bearing residues were mounted on slides using glycerin jelly, and were sealed with paraffin wax. At least c. 250 sporomorphs were counted per sample. All samples were studied using an Olympus BX41 microscope. Photomicrographs were taken using a Zeiss Imager Z2 microscope and an AxioCam HRc imaging system. The SMG (Spore-pollen Morphological Group) method outlined by Visscher & Van der Zwan (Reference Visscher and Van der Zwan1981) and the SEG (Sporomorph EcoGroup) model established by Abbink (Reference Abbink1998) and Abbink, van Konijnenburg-van Cittert & Visscher (Reference Abbink, van, Cittert and Visscher2004) were applied in this study to reconstruct the palaeoclimatic variations. All palynological slides are stored at the Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, Nanjing, China.
4. Palynology
The miospores of the Xujiahe Formation in the Hechuan Section of Chongqing City, southern Sichuan Basin are diverse and well preserved, represented by 184 species of spores and pollen in 75 genera (see online Supplementary Table S1, available at http://journals.cambridge.org/geo). The representative miospores are illustrated in Figures 3–5. The palynoflora of the Xujiahe Formation from the Hechuan region has previously been assigned a Norian–Rhaetian age (Liu, Li & Wang, Reference Liu, Li and Wang2015b), which is supported by a recent geomagnetic study (Li et al. Reference Li, Zhang, Huang, Ogg, Hinnov, Wang, Zou and Li2017). Here we provide a more detailed vegetation reconstruction coupled with palaeoclimatic interpretations through the studied succession. For palaeoclimatic and palaeoecological purposes, the palynological assemblages were divided based on relative abundance. Three assemblages were recognized and the percentages of selected taxa are illustrated in Figure 6. The characteristic features for each assemblage are outlined below in ascending stratigraphic order. The percentages are expressed in whole numbers.

Figure 3. Representative spore taxa recovered from the Xujiahe Formation of the Hechuan region. Taxa names are followed by slide number. (a–c) Dictyophyllidites harrisii: (a, b) HC10-1; (c) HC11-3. (d) Dictyophyllidites mortonii, HC26-6. (e–h) Concavisporites toralis: (e) HC10-4; (f–h) HC9-1. (i) Cyathidites australis, HC28-2. (j, k) Cyathidites minor: (j) HC13-2; (k) HC18-4. (l) Punctatisporites triassicus, HC13-1. (m) Leiotriletes adnatus, HC13-2. (n) Leiotriletes toroiformis, HC26-1. (o) Toroisporis sp., HC10-6. (p) Osmundacidites wellmanii, HC13-1. (q) Lunzisporites lunzensis, HC30-2. (r, t) Anapiculatisporites spiniger: (r) HC11-6; (t) HC24-6. (s) Lophotriletes sparsus, HC18-2. (u) Granulatisporites granulatus, HC13-1. (v, w) Acanthotriletes aculeatus: (v) HC17-3; (w) HC18-4. (x, ee) Asseretospora gyrata, HC13-3. (y, z) Annulispora folliculosa: (y) HC11-2; (z) HC13-1. (aa) Lycopodiacidites rudis, HC11-4. (bb) Lycopodiumsporites sp., HC13-5. (cc) Asseretospora curvata, HC13-1. (dd) Kyrtomisporis laevigatus, HC10-2. (ff) Asseretospora scanicus, HC13-3.

Figure 4. Representative miospore taxa recovered from the Xujiahe Formation of the Hechuan region. Taxa names are followed by slide number. (a, b) Kyrtomisporis speciosus: (a) HC17-4; (b) HC21-6. (c) Kraeuselisporites punctatus, HC11-1. (d, h) Aratrisporites scabratus: (d) HC13-6; (h) HC20-3. (e, f) Araucariacites australis: (e) HC26-5; (f) HC18-2. (g) Chasmatosporites apertus, HC11-1. (i) Chasmatosporites hians, HC32-5. (j) Cycadopites reticulata, HC7-3. (k) Cycadopites pyriformis, HC24-1. (l) Cycadopites typicus, HC20-1. (m) Cycadopites deterius, HC28-2. (n) Chasmatosporites major, HC18-2. (o, q) Monosulcites minimus: (o) HC11-2; (q) HC13-2. (p) Monosulcites enormis, HC31-5. (r) Monosulcites fusiformis, HC18-3. (s, t) Classopollis minor: (s) HC32-5; (t) HC25-1. (u) Uvaesporites sp., HC31-4. (v) Ovalipollis ovalis, HC11-4.
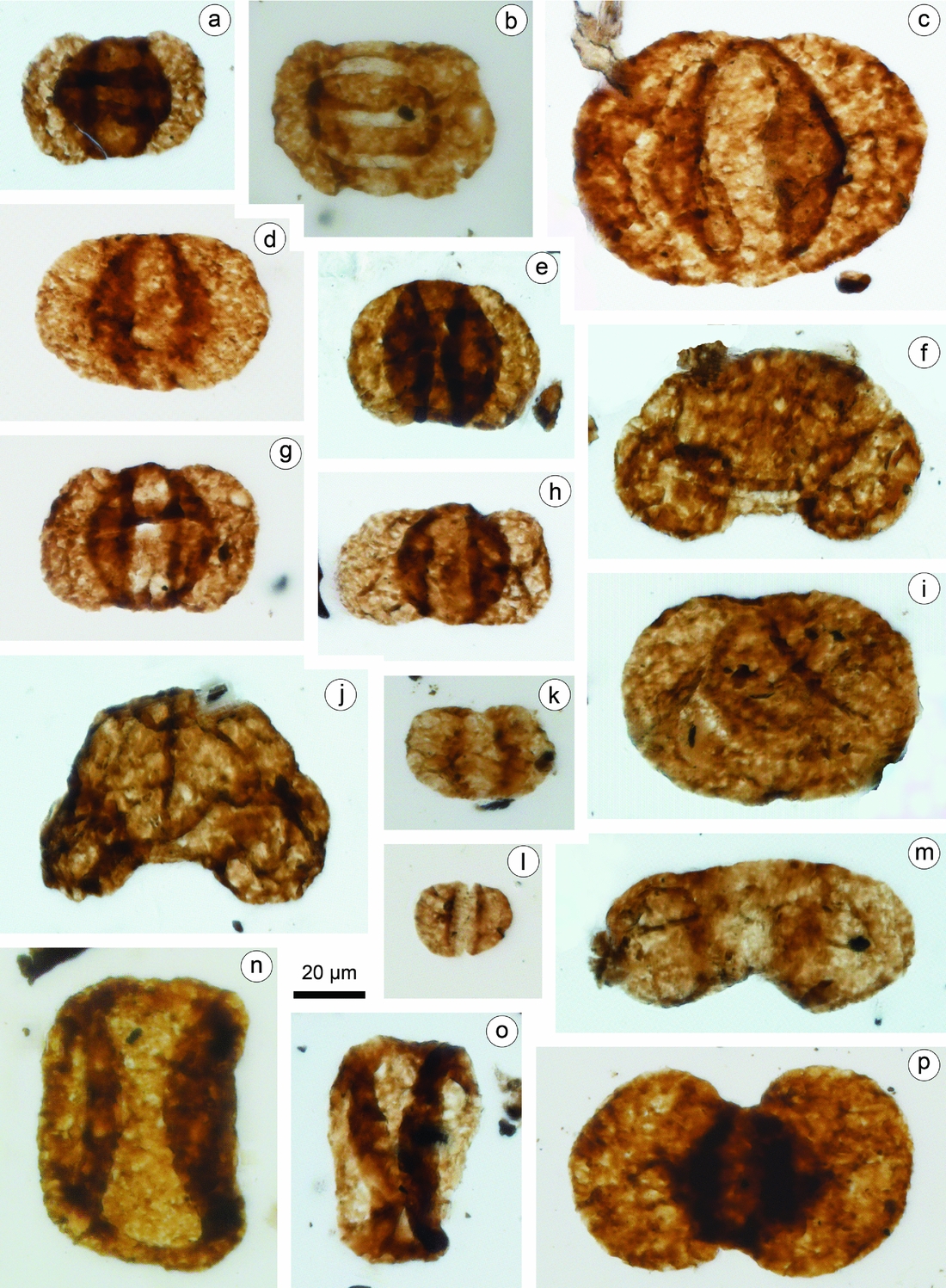
Figure 5. Representative pollen taxa recovered from the Xujiahe Formation of the Hechuan region. Taxa names are followed by slide number. (a) Lueckisporites triassicus, HC13-2. (b) Taeniaesporites noviaulensis, HC13-5. (c) Alisporites australis, HC13-1. (d) Alisporites parvus, HC13-1. (e) Alisporites bilateralis, HC13-2. (f) Pinuspollenites divulgatus, HC13-2. (g, h) Pinuspollenites enodatus, HC13-1. (i) Paleoconiferus asaccatus, HC13-2. (j) Pinuspollenites alatipollenites, HC13-2. (k, l) Vitreisporites pallidus: (k) HC13-4; (l) HC13-1. (m) Podocarpidites multisimus, HC13-2. (n, o) Quadraeculina anellaeformis: (n) HC21-1; (o) HC11-3. (p) Platysaccus queenslandi, HC13-4.

Figure 6. Abundance diagram of major spore-pollen genera and assemblages represented in the samples from the Xujiahe Formation in the Tanba Section of the Hechuan region.
4.a. Dictyophyllidites harrisii – Concavisporites toralis – Kyrtomisporis laevigatus – Aratrisporites fischeri (DCKA) assemblage (samples HC07–HC10)
The DCKA assemblage is identified in Member I and within the base of Member III of the Xujiahe Formation (Fig. 6). It is characterized by a significant dominance of spores (average 77%), highly dominated by trilete fern spores (mostly produced by ground ferns) represented mainly by Concavisporites/Dictyophyllidites (23%), followed by Leiotriletes (8%), Kyrtomisporis (7%), Granulatisporites (6%), Cyathidites (4.5%) and Toroisporis (3%). Other spore genera occurring in lower abundances (1–3%) within this assemblage include Uvaesporites, Klukisporites, Lunzisporites, Punctatisporites, Planisporites, Asseretospora, Sphagnumsporites, Anapiculatisporites and Kraeuselisporites. Monolete spores comprise >5% and are mainly represented by Aratrisporites produced by lycophytes (Fig. 6; Table 1).
Table 1. Botanical affinity and classification of the Sporomorph EcoGroup (SEGs) for dispersed miospores of the Xujiahe Formation in the Hechuan region, southern Sichuan Basin, China

Note: This summary is based upon comprehensive results of the in situ spore studies of the Mesozoic plants and their ecology based on Couper (Reference Couper1957); Harris (Reference Harris1961, Reference Harris1964, Reference Harris1969, Reference Harris1979), Pocock & Jansonius (Reference Pocock and Jansonius1969), van Konijnenburg-van Cittert (Reference van Konijnenburg-van Cittert1971, Reference van Konijnenburg-van Cittert1978, Reference van Konijnenburg-van Cittert1993, Reference van Konijnenburg-van Cittert2002), Litwin (Reference Litwin1985), Osborn & Taylor (Reference Osborn and Taylor1993), Balme (Reference Balme1995), Wang & Mei (Reference Wang and Mei1999), Deng & Chen (Reference Deng and Chen2001), Abbink, van Konijnenburg-van Cittert & Visscher (Reference Abbink, van, Cittert and Visscher2004), Wang, Mosbrugger & Zhang (Reference Wang, Mosbrugger and Zhang2005), Jiang et al. (Reference Jiang, Wang, Robbins, Wei and Tian2008), Guignard et al. (Reference Guignard, Wang, Ni, Tian and Jiang2009), Wang & Zhang (Reference Wang and Zhang2010) and Wang et al. (Reference Wang, Li, Guignard, Dilcher, Xie, Tian, Zhou and Wang2015).
In the DCKA assemblage, gymnosperm pollen grains reach an average of 24% which are dominated by monocolpate pollen grains (average 11%, including Chasmatosporites, Cycadopites and Monosulcites). Bisaccate conifer pollen grains reach average relative abundance of 6% (including Pinuspollenites, Protopinus, Piceites, Pseudopicea, Podocarpidites, Quadraeculina, Protoconiferus, Chordasporites and Taeniaesporites). Bisaccate seed fern pollen (including Alisporites and Vitreisporites) are less abundant (c. 2%) (Fig. 6).
4.b. Cycadopites reticulata – Pinuspollenites divulgatus – Dictyophyllidites harrisii – Aratrisporites fischeri (CPDA) assemblage (samples HC11–HC22)
The CPDA assemblage occurs in the upper part of Member III of the Xujiahe Formation (Fig. 6). It is characterized by the predominance of gymnosperm pollen grains (average relative abundance of 56%) and a significantly lower portion of spores (44%) compared to the other two assemblages.
Monocolpate pollen grains dominate (average of c. 26%), represented by Cycadopites (11%), Chasmatosporites (10%) and Monosulcites (5%). Bisaccate conifer pollen grains are the second-most abundant type (average 15%), represented by Pinuspollenites (7%), Paleoconiferus (2%), Piceites (2%), Quadraeculina (2%) and Pseudopicea (1%). Bisaccate seed fern pollen (including Alisporites and Vitreisporites) and Araucariacites increase in abundance (7% and 6%, respectively). Classopollis is rare (0.5%), and shows a distinct increase in the uppermost part of this assemblage (Fig. 6).
Trilete spores are the dominant type among spores in the CPDA assemblage (average 39%), marked mainly by Concavisporites/Dictyophyllidites (10%), Cyathidites (6%) and Granulatisporites (4%). Other spore genera are common (1–3%) in this assemblage such as Asseretospora, Osmundacidites, Lophotriletes, Acanthotriletes, Conbaculatisporites, Cyclogranisporites, Punctatisporites and Kyrtomisporis. Monolete spores are also common (5%), and are mainly represented by Aratrisporites produced by lycophytes (4%) (Fig. 6).
4.c. Cyathidites minor – Monosulcites fusiformis – Classopollis minor – Quadraeculina anellaeformis (CMCQ) assemblage (samples HC24–HC32)
The assemblage CMCQ occurs in Members IV–VI of the Xujiahe Formation (Fig. 6) and is characterized by a significant dominance of gymnosperm pollen grains (relative abundance 64%). Monosulcate pollen (Monosulcites, Cycadopites and Chasmatosporites) are the most prominent type. In comparison with the other two assemblages, a higher portion is represented by pollen attributed to seed ferns (8%). The relatively high abundance of Classopollis (6%) is also significant, a taxon that is virtually absent from the other two assemblages. Spores make up 36% with a significant dominance of Cyathidites (10%). Monolete spores are rare, comprising <1% (Fig. 6).
5. Development of the vegetation
Based on the botanical affinity of the dispersed spore and pollen genera recovered from the studied successions within the Hechuan region of the Sichuan Basin (Table 1), a picture of a diverse Late Triassic ecosystem emerges. Although the vegetation is chiefly dominated by ferns and conifers, other plant groups are present in lower relative abundance. These include mosses, horsetails and lycopsids which vary considerably in relative abundance through the studied succession (Fig. 7). The overall evolution of the Late Triassic vegetation in the Hechuan region is suggested to have undergone changes from lowland fern forest to a mixed forest with more canopy trees.
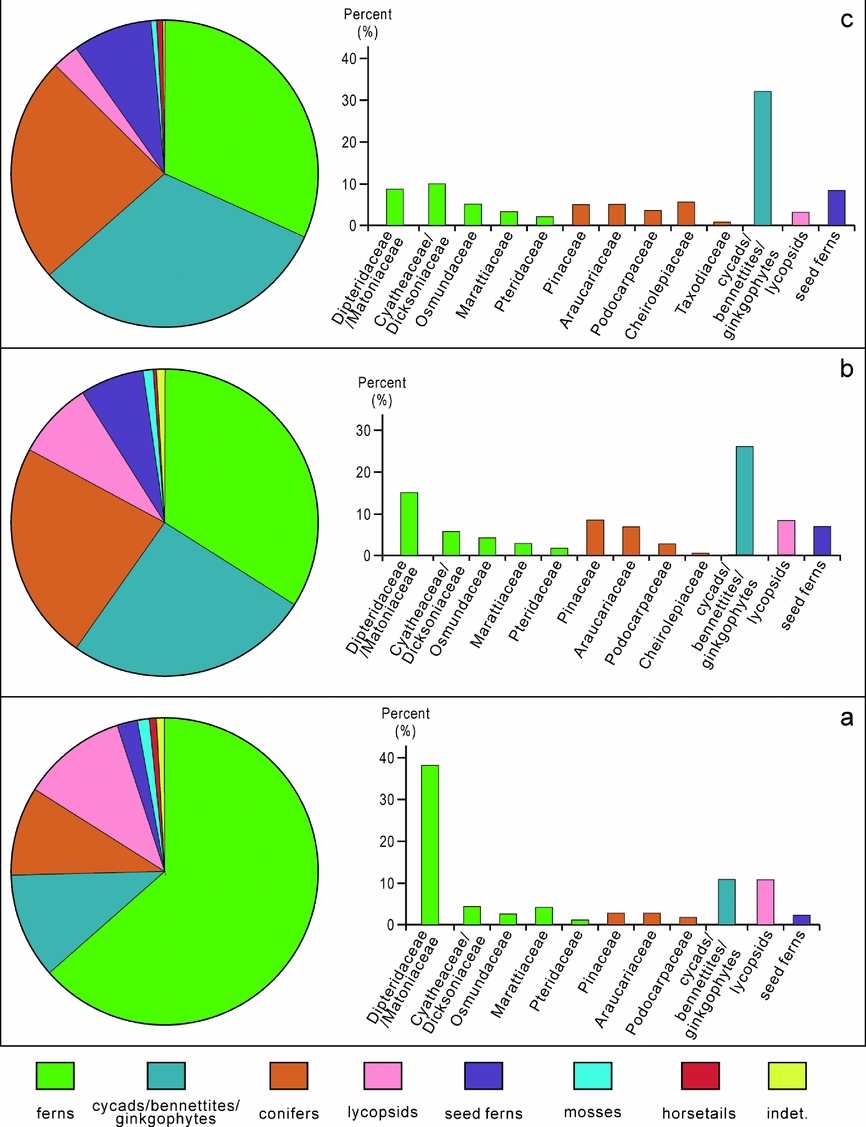
Figure 7. Palaeovegetation composition of the Xujiahe Formation from the Hechuan region. (a) DCKA assemblage (Member I and base of Member III); (b) CPDA assemblage (Member III); and (c) CMCQ assemblage (Members IV, V, VI).
The earliest Late Triassic DCKA ecosystem (represented in Member I and the base of Member III) was dominated by ferns, mainly Dipteridaceae/Matoniaceae together with a variety of other fern families including Cyatheaceae/Dicksoniaceae, Osmundaceae and Marattiaceae. These ferns, together with typical Triassic lycopsids and rare mosses, comprised the ground cover vegetation during the earliest part of Late Triassic time. The mid-storey was represented by gymnosperms related to cycads/bennettites/ginkgophytes. Canopy trees were relatively scarce, represented by Pinaceae, Araucariaceae and Podocarpaceae, and the pollen may have been transported in from elevated areas into the lowlands. Seed ferns existed, but made up a very limited portion of this ecosystem.
The CPDA ecosystem was characterized by dominance in gymnosperms, mainly represented by cycads/bennettites/ginkgophytes making up the mid-storey bush vegetation together with seed ferns. Canopy vegetation was represented by relatives of Pinaceae. The ferns are much less prominent in the CPDA assemblage compared to the older DCKA assemblage (Fig. 7b), and these were mainly represented by Dipteridaceae/Matoniaceae. Lycopsids are not as abundant compared with the DCKA assemblage. A new element, the family Cheirolepidiaceae (Classopollis), a group that was common during Jurassic and Early Cretaceous time around the world (Alvin, Reference Alvin1982; Vajda, Reference Vajda2001; Vajda & Wigforss-Lange, Reference Vajda and Wigforss-Lange2006; Jansson et al. Reference Jansson, McLoughlin, Vajda and Pole2008), interestingly appears in this assemblage.
In assemblage CMCQ, represented within the upper part of the Xujiahe Formation (Member V and VI), cycads/bennettites/ginkgophytes and conifers characterize the flora. It is interesting to note that Cyatheaceae/Dicksoniaceae show a sharp increase in abundance (average 10%). Other fern families include Dipteridaceae/Matoniaceae, Osmundaceae, Marattiaceae and Pteridaceae. The mid-storey vegetation was dominated by monosulcate pollen producers, cycads/bennettites/ginkgophytes (Fig. 7c). Conifers including Cheirolepidiaceae, Araucariaceae, Pinaceae, Podocarpaceae and Taxodiaceae were prominent (average 24%), making up the canopy. It is notable that Cheirolepidiaceae shows a remarkable increase in relative abundance and becomes common during this period (average 6%). Seed ferns show an increasing trend (8%). Lycopsids are less frequent, and mosses and horsetails are rare.
6. Palaeoclimatic interpretations
As a complement to the vegetation reconstruction based on the abundance data of pollen and spores related to their affinities, we have carried out palaeoclimatic interpretations by applying the Spore-pollen Morphological Group (SMG) method (Visscher & Van der Zwan, Reference Visscher and Van der Zwan1981) and the Sporomorph EcoGroup model (SEG) (Abbink, Reference Abbink1998; Abbink, van Konijnenburg-van Cittert & Visscher, Reference Abbink, van, Cittert and Visscher2004).
Twelve Spore-pollen Morphological Groups (SMGs) A–L were identified in this study (Fig. 8), reflecting different ecological adaptations, including hygrophytic (water-loving, groups A–D), xerophytic (dry-loving, groups H–L) and intermediate elements (groups E–G). We have applied the ratio of hygrophytic elements to xerophytic elements (hygrophytic/xerophytic) as an index of humidity variation. The results (Fig. 8) show that the hygrophytic/xerophytic ratio is high in the lowermost part of the Xujiahe Formation, particularly at the base of Member III (Fig. 8, line A), indicating a humid pulse of short duration and expressed in one sample within the Xujiahe Formation. This is in agreement with the results based on the vegetation composition in Assemblage DCKA. The hygrophytic/xerophytic ratio is markedly low for the rest of the succession (with the exception for sample HC16; Fig. 8, line B), suggesting a drying trend upwards, interrupted by a short humid pulse.

Figure 8. Relative abundances of the Spore-pollen Morphological Groups (SMGs) of the Xujiahe Formation from the Hechuan region. A, Trilete acavate laevigate or apiculate spores; B, Trilete acavate reticulate or murornate spores; C, Trilete cingulate or zonate spores; D, Monolete spores; E, Ovalipollis+Perinopollenites; F, Monosulcate pollen; G, Asaccate pollen; H, Monosaccate pollen; I, Trilete (proto) bisaccate pollen; J, Alete bisaccate pollen; K, Taeniate (proto) bisaccate pollen; L, Classopollis spp. A–D are considered to be hygrophytic elements, E–G intermediate and H–L xerophytic elements.
Using the Sporomorph EcoGroup model (SEG) (Abbink, Reference Abbink1998; Abbink, van Konijnenburg-van Cittert & Visscher, Reference Abbink, van, Cittert and Visscher2004), we classified the palynomorphs into three SEG groups, including: (1) Lowland SEG; (2) Upland SEG; and (3) River SEG (Table 1, Fig. 9). Elements attributed to the Lowland SEG show a marked dominance in the Xujiahe Formation, with a maximum of 81% and a minimum of 46%; the River SEG and Upland SEG are less abundant. The total for the Lowland SEG and River SEG is a minimum of 69% (Fig. 9). This implies that the studied area during Late Triassic time was represented by a general lake-marsh environment set in a lowland ecosystem. However, variations in the ecosystem and the climate during Late Triassic time are reflected in the palynological assemblages of this study, revealing that the ecosystem was not constant throughout this period as previously suggested (Huang & Lu, Reference Huang and Lu1992; Wang et al. Reference Wang, Kan, Liu, Liang and Zhu2008, Reference Wang, Fu, Xie, Huang, Li, Li, Liu, Yu, Pan, Tian and Jiang2010).

Figure 9. Relative abundances of the Sporomorph EcoGroups (SEGs) of the Xujiahe Formation from the Hechuan region.
As indicated by the hygrophytic elements (groups A–D; Fig. 8), the highest abundance of the Lowland SEG is found in the lowermost sample of Member III (Fig. 9, line A), corresponding to the highest values of the Lowland/Upland ratio and Lowland wet/dry ratio, the lowest value of the Upland SEG and a relatively high warm/cool ratio, suggesting a relatively warm and humid interval. The Lowland wet/dry ratio is only high in the lowermost two samples, and shows a striking upwards decreasing trend, suggesting a general drying trend. The Lowland SEG and the Lowland/Upland ratio show two smaller peaks at samples HC16 of Member III (Fig. 9, line B) and HC24 of Member IV (Fig. 9), indicating two short intervals of humid climate conditions. The Lowland warm/cool ratio shows an overall decreasing upwards trend in the Xujiahe Formation, but has a striking peak at sample HC25 of Member IV (Fig. 9, line C), indicating an overall cooling trend during the Late Triassic period interrupted by a short warmer climate interval.
Previous palynological and palaeobotanical studies from the Sichuan Basin have shown that the Late Triassic palaeoclimate was generally one of humid and warm tropics-subtropics (Huang & Lu, Reference Huang and Lu1992; Wang et al. Reference Wang, Kan, Liu, Liang and Zhu2008, Reference Wang, Fu, Xie, Huang, Li, Li, Liu, Yu, Pan, Tian and Jiang2010). However, with our more detailed, high-resolution palynological study, a different picture emerges. Our data reveal a highly variable Late Triassic ecosystem represented by a warm and humid climate during the earliest Late Triassic period (Member I and base of Member III), followed by a cooler and drier interval interrupted by two wetter and one warmer episodes.
7. Discussion
Our palynological study indicates an overall cooling and drying trend during latest Norian–Rhaetian time, accompanied by a general decrease in ferns (mainly represented by trilete spores), an increase in gymnosperms (represented by bisaccate and monocolpate pollen), and a decline in diversity of both pollen and spores (Fig. 10). Similar results have been reported from coeval deposits in Xuanhan, northeastern Sichuan Basin, indicating a cooling and drying climate during the development of the uppermost part of the Xujiahe Formation (Li et al. Reference Li, Wang, Liu, Zhou and Wang2016). The above outlined climate change is consistent with macrofloral studies of the Xujiahe Formation, which also implied a palaeoclimatic trend from humid to arid conditions (Huang & Lu, Reference Huang and Lu1992). Palynological records from northwestern and central Europe, Western Australia and northeastern Greenland revealed a cooling during latest Triassic time (Hubbard & Boulter Reference Hubbard and Boulter1997, Reference Hubbard and Boulter2000) and the trend from humid to arid has also been noted from the Newark Basin (Kent & Olsen, Reference Kent and Olsen2000; Olsen & Kent, Reference Olsen, Kent, Bachmann and Lerche2000) where it has been linked to the northwards drift of the North American continent. Palynological data from Austria and the United Kingdom indicated a warming trend from the Triassic to the Jurassic periods, interrupted by a cooler period (Bonis & Kürschner, Reference Bonis and Kürschner2012). Further, a bentho-planktonic study from the Austrian Alps suggested that cooling episodes might have occurred during latest Triassic time (Clémence et al. Reference Clémence, Gardin, Bartolini, Paris, Beaumont and Guex2010). The above results may suggest a global cooling event during latest Triassic time. Tucker & Benton (Reference Tucker and Benton1982) proposed climate-induced (increasing aridity) floral changes as a factor in Late Triassic tetrapod extinction. The present palynological record seems more consistent with a gradual ecosystem degradation extended over the Norian–Rhaetian interval. The cooling and drying climate from latest Norian to Rhaetian time may have caused a gradual ecosystem breakdown during latest Triassic time, and later triggered the end-Triassic biotic crisis.

Figure 10. Palaeoclimate, miospore composition and miospore diversity of the Xujiahe Formation from the Hechuan region.
8. Conclusions
Our detailed palynological investigation of Upper Triassic terrestrial deposits within the Sichuan Basin has revealed a well-preserved and diverse palynoflora.
(1) Our study reveals an ecosystem in change where a fern-dominated vegetation was replaced by conifers and cycadoids, supplemented by relative high portions of Classopollis in the uppermost Triassic strata. Palynological diversity patterns show a decreasing trend upsection.
(2) Three palynological assemblages were distinguished by variations in the abundance of major plant groups, reflecting remarkable changes in the terrestrial vegetation throughout the entire interval. Cycads/bennettites/ginkgophytes and conifers show an increasing trend into younger deposits, while ferns and lycopsids decrease in relative abundance.
(3) By applying the SMG method and SEG model analysis, we show that the early stage of the Late Triassic period was characterized by a relatively warm and humid climate which was followed by a cooler and drier interval. This demonstrates that the climate was not static, but rather variable.
(4) Our results reveal vegetation changes within the Sichuan Basin during the Late Triassic Period, adding to knowledge on biotic changes immediately prior to the end-Triassic event.
Acknowledgements
We acknowledge Xiaoping Xie, Ning Tian, Ning Zhou, Shucheng Xie, Mingsong Li and Ms Feng Limei for field and laboratory assistance. This work was financially supported by the Strategic Priority Research Program (B) of the Chinese Academy of Sciences (XDB18000000, XDPB0506); the National Natural Sciences Foundation of China (NSFC 41572014, 41688103); the State Key Program of Research and Development of Ministry of Science and Technology, China (2016YFC0600406); the State Key Laboratory of Palaeobiology and Stratigraphy (20172103); the Swedish Research Council (VR 2015–04264) and the Lund University Carbon Cycle Centre (LUCCI). This is a contribution to the IGCP project 632, sponsored by Unesco/IUGS. We also thank Evelyn Kustatscher and an anonymous reviewer for their constructive comments which led to the improvement of the manuscript.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0016756817000735.













