Highlights
-
HREE mineralization occurs in fenitized phonolite breccia, related to carbonatite.
-
Mineralization consists of xenotime, zircon, anatase/rutile and minor huttonite/thorite.
-
Mineralization shares textural and compositional similarity to higher-grade HREE occurrences in carbonatite and lower-grade occurrences in fenite.
-
Carbonatite-derived, HREE-rich residual fluids are focused through high-permeability fenite breccia pipes.
-
HREE and Th in fenite breccias may indicate the presence of LREE mineralization in a carbonatite at depth.
1. Introduction
The rare earth elements (REE) exhibit magnetic and spectroscopic properties useful in a number of technological and industrial applications, and demand for REE is increasing (Goodenough et al. Reference Goodenough, Wall and Merriman2018). Carbonatites host some of the largest metallurgically-favourable REE resources (Wall, Reference Wall and Gunn2014; Verplanck et al. Reference Verplanck, Mariano and Mariano2016), yet the REE minerals commonly extracted from carbonatites (REE fluorcarbonates, monazite) are typically light (L)REE-rich (La–Sm) and heavy (H)REE-poor (Eu–Lu + Y; Wall, Reference Wall and Gunn2014). With the exception of Nd and Pr, they are, therefore, deficient in many REE which are of economic importance but at risk of supply disruption, such as Dy and Tb (European Commission, 2020: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52020DC0474). The supply imbalance between LREE and HREE is termed the ‘balance problem’ (Binnemans et al. Reference Binnemans, Jones, Müller and Yurramendi2018), and is a major cause of undersupply of HREE and, thus, the high price of these elements.
The high value of the HREE makes carbonatites with some degree of HREE enrichment particularly attractive for exploitation. Only a few examples of carbonatite-related HREE enrichment are known, and include: Lofdal, Namibia (Wall et al. Reference Wall, Niku-Paavola, Storey, Müller and Jeffries2008; Bodeving et al. Reference Bodeving, Williams-Jones and Swinden2017); Chilwa Island, Kangankunde, Songwe and Tundulu, Malawi (Ngwenya, Reference Ngwenya1994, Wall & Mariano, Reference Wall, Mariano, Jones, Wall and Williams1996; Broom-Fendley et al. Reference Broom-Fendley, Styles, Appleton, Gunn and Wall2016a, b, Reference Broom-Fendley, Brady, Wall, Gunn and Dawes2017a, b, c; Dowman et al. Reference Dowman, Wall, Treloar and Rankin2017a); Salpeterkop, South Africa (Verwoerd et al. Reference Verwoerd, Viljoen and Chevallier1995); Pivot Creek, New Zealand (Cooper et al. Reference Cooper, Collins, Palin and Spratt2015); Huanglongpu and Huayangchuan, China (Xu et al. Reference Xu, Campbell, Allen, Huang, Qi, Zhang and Zhang2007, Reference Xu, Kynicky, Chakhmouradian, Campbell and Allen2010; Song et al. Reference Song, Xu, Smith, Kynicky, Huang, Wei, Zhou and Shu2016; Smith et al. Reference Smith, Kynicky, Xu, Song, Spratt, Jeffries, Brtnicky, Kopriva and Cangelosi2018; Cangelosi et al. Reference Cangelosi, Smith, Banks and Yardley2020a); and Bear Lodge, USA (Andersen et al. Reference Andersen, Clark, Larson and Neill2016, Reference Andersen, Clark, Larson and Donovan2017). These examples vary from mineral-scale enrichment of academic interest, to deposits of sufficient size to potentially constitute ore.
One of the best-known examples of carbonatite-related HREE enrichment is the Lofdal deposit, Namibia. Here, the principal REE ore mineral is xenotime-(Y) ([Y, HREE]PO4), which occurs in albitized fault zones radiating from a central carbonatite complex (Wall et al. Reference Wall, Niku-Paavola, Storey, Müller and Jeffries2008; HS Swinden & P Siegfried, unpub. technical report, 2011; Do Cabo, Reference Do Cabo2013; Loye, Reference Loye2014; Bodeving et al. Reference Bodeving, Williams-Jones and Swinden2017). While the nature of the carbonate units in the fault zones is debated (e.g. Wall et al. Reference Wall, Niku-Paavola, Storey, Müller and Jeffries2008; Do Cabo, Reference Do Cabo2013; Williams-Jones et al. Reference Williams-Jones, Wollenberg, Bodeving, Simandl and Neetz2015; Bodeving et al. Reference Bodeving, Williams-Jones and Swinden2017), the coeval nature of the xenotime with the surrounding carbonatite rocks indicates a genetic relationship (Wall et al. Reference Wall, Niku-Paavola, Storey, Müller and Jeffries2008). Similarly, at the Bear Lodge carbonatite complex, USA, HREE minerals occur c. 2 km from the main intrusion centre at the Cole high-field-strength elements (HFSE) (+HREE) occurrence (Andersen et al. Reference Andersen, Clark, Larson and Neill2016). Here, xenotime-(Y) (and anatase) occur in recrystallized quartz and K-feldspar-rich breccia, along the contacts of sedimentary units. Based on mineral textures, Andersen et al. (Reference Andersen, Clark, Larson and Neill2016) interpret the xenotime-(Y) to be causally linked to carbonatite emplacement. While mineral-scale fractionation of the REE has been demonstrated at many carbonatites (Broom-Fendley et al. Reference Broom-Fendley, Styles, Appleton, Gunn and Wall2016a, Reference Broom-Fendley, Brady, Wall, Gunn and Dawes2017a; Andersen et al. Reference Andersen, Clark, Larson and Donovan2017), the occurrences at Lofdal and Bear Lodge show that fractionation of the REE also occurs on the deposit scale. However, it remains unclear if these examples are an exception, or if HREE mineralization around carbonatites is a common, previously overlooked, feature.
Alkali metasomatism is present at both the Cole occurrence (Bear Lodge) and the Lofdal deposit. At the Cole occurrence, HREE and HFSE minerals are associated with abundant K-feldspar growth, while xenotime at Lofdal is associated with albite. Alkali metasomatic aureoles, termed fenites, are common around carbonatites, as bodies of carbonatite expel alkali-rich fluids as they cool and crystallize. Such fluids metasomatize country rock, removing silica and adding alkalis (Na2O + K2O) (Le Bas, Reference Le Bas2008; Elliott et al. Reference Elliott, Wall, Chakhmouradian, Siegfried, Dahlgren, Weatherley, Finch, Marks, Dowman and Deady2018). A potassic fenite ring and carapace forms proximal to the intrusion and is typically highly brecciated, consisting of country rock that is pervasively altered to high concentrations of K-feldspar and iron and manganese oxides. An outer ring of sodic fenite can extend up to 2 km from the source, predominantly consisting of sodic amphibole or clinopyroxene veins in the country-rock host (see Elliott et al. Reference Elliott, Wall, Chakhmouradian, Siegfried, Dahlgren, Weatherley, Finch, Marks, Dowman and Deady2018 and references therein). Many fenites contain localized REE enrichment resulting from the transport of these elements from the carbonatite melt by fenitizing fluids. For example, fenite at the Alnö Complex, Sweden, is REE-enriched by 49–354 ppm in the potassic zone and 0–807 ppm in the sodic zone, compared to the original migmatite protolith (Morogan, Reference Morogan1989). REE-enriched micro-mineral assemblages (fine-grained mineral assemblages containing micron-scale REE minerals) occur in fenite at Meech Lake, Québec (Hogarth, Reference Hogarth2016), and Chilwa Island, Malawi (Dowman et al. Reference Dowman, Wall, Treloar and Rankin2017a). The micro-mineral assemblages are predominantly limited to LREE minerals, but these exhibit elevated HREE contents compared to their carbonatite-hosted counterparts. Extremely fenitized rocks can host xenotime in small amounts (Dowman et al. Reference Dowman, Wall, Treloar and Rankin2017a).
In this contribution, we describe HREE-enriched occurrences of fenitized phonolite breccia from the Chilwa Alkaline Province, Malawi, which share textural and geochemical similarities to the Lofdal deposit and Cole occurrence. The breccias reported here occur on the periphery of the Songwe Hill carbonatite and are directly adjacent to the Mauze nepheline syenite complex. We also present data on breccias from the Nkalonje carbonatite complex which are similar in terms of their texture and major element composition, but do not exhibit HREE enrichment.
2. Geological background and field observations
The late Jurassic – early Cretaceous Chilwa Alkaline Province of southern Malawi and Mozambique consists of alkali granite, nepheline syenite and numerous carbonatite complexes (Fig. 1; Woolley & Garson, Reference Woolley, Garson and Clifford1970; Woolley, Reference Woolley, Kampunzu and Lubala1991, Reference Woolley2001). Of the carbonatites, Kangankunde, Tundulu and Songwe Hill host REE deposits of economic interest (Ngwenya, Reference Ngwenya1994; Wall & Mariano, Reference Wall, Mariano, Jones, Wall and Williams1996; Broom-Fendley et al. Reference Broom-Fendley, Brady, Wall, Gunn and Dawes2017a, b). At present, however, only Songwe Hill is being developed and has a combined measured and indicated mineral resource estimate of 21 Mt, grading 1.41 % TREO (total rare earth oxides; C Witley et al., unpub. technical report, 2020). At many Chilwa carbonatite complexes, the carbonatite itself is a minor part of an exposed complex. Instead, these complexes are distinguished by large, near-circular, breccia units composed of heavily altered K-feldspar-rich rock, termed feldspathic breccia (Garson, Reference Garson1965). Feldspathic breccias from the Songwe Hill / Mauze and Nkalonje complexes are the focus of this work.

Fig. 1. (a) Map of the Chilwa Alkaline Province, highlighting the locations of the major carbonatites. Adapted from Woolley (Reference Woolley2001) and Broom-Fendley et al. (Reference Broom-Fendley, Brady, Wall, Gunn and Dawes2017a). (b) Regional geological map with the study areas marked in boxes, redrawn after Garson & Walshaw (Reference Garson and Walshaw1969). Sample locations at Nkalonje are marked by red circles.
2.1 The Songwe Hill carbonatite and the Mauze nepheline syenite complexes
The Songwe Hill carbonatite complex abuts the larger Mauze nepheline syenite (Fig. 2a). Songwe Hill predominantly comprises fenitized and, locally, extensively brecciated fine-grained alkaline silicate rock, into which at least three different carbonatite stages have been emplaced (Broom-Fendley et al. Reference Broom-Fendley, Brady, Wall, Gunn and Dawes2017a). The different carbonatite stages principally consist of fine-grained calcite carbonatite (C2) and a more Sr- and REE-rich ferroan variety (C3); coarse-grained calcite carbonatite (C1) is volumetrically insignificant at surface but occurs as clasts in later carbonatite units. Late-stage Fe- and Mn-rich veins and apatite fluorite veins cut all carbonatite stages, and the complex has undergone several stages of hydrothermal alteration and recrystallization (Broom-Fendley et al. Reference Broom-Fendley, Heaton, Wall and Gunn2016b). Notably, apatite at the complex is abnormally HREE-rich, for a carbonatite, and apatite–fluorite veins contain minor xenotime-(Y) (Broom-Fendley et al. Reference Broom-Fendley, Brady, Horstwood, Woolley, Mtegha, Wall, Dawes and Gunn2017b). Both the HREE-enriched apatite and xenotime are considered to be late hydrothermal phases based on fluid inclusion microthermometry of associated fluorite (Broom-Fendley et al. Reference Broom-Fendley, Heaton, Wall and Gunn2016b, Reference Broom-Fendley, Brady, Horstwood, Woolley, Mtegha, Wall, Dawes and Gunn2017b).

Fig. 2. (a) Geological map of the Songwe/Mauze complex, showing the location of the altered breccia vents and samples. (b) Radiometric colour map of Th concentration, courtesy of Mkango Resources. Red: higher Th; blue: lower Th (arbitrary units). Note the elevated Th contents at the altered breccia vents. Coordinate system is UTM 36S, WGS1984 datum.
Recent mapping has focused on the wider relationship between Songwe and the surrounding Mauze nepheline syenite (Fig. 2a). Several small breccia units occur at the contact between Mauze and the surrounding basement gneiss, and these are the main focus of this study. The breccia units to the south of Songwe are colloquially termed ‘Mantrap’, while those to the west are termed the ‘School vents’ and those to the northeast are the ‘North vents’. Both the School vents and those at Mantrap correspond to areas of high Th counts in radiometric surveys (Fig. 2b), which aided their discovery.
The breccia units at the Songwe Hill / Mauze complex are located at, or close to, the contact between nepheline syenite and the surrounding country rock (Fig. 2a). They are small, the largest comprising an area no more than ˜125 × 125 m, and occur at the top of small hills abutting the larger, steeper, Mauze mountain (Fig. 3). Exposure is poor, but the boundaries of the breccia units can be roughly demarked by the extent of reddish-brown, slightly radioactive, soils.

Fig. 3. Annotated field photo showing the morphology of the breccia, and its association with the Mauze nepheline syenite. Photo location indicated in Figure 2.
Owing to sparse outcrop, many of the available samples are float (Fig. 2a). On the weathered surface, the rocks are buff–pink in colour, with local black Mn-oxide staining (Fig. 4a). Most samples are heavily altered and composed predominantly of clay minerals, after K-feldspar, and Fe- and Mn-oxide phases (Fig. 4b). The rocks contain varying proportions of sub-rounded clasts of various protoliths. Clasts range from small, mm-scale fragments to c. 50 cm across. A K-feldspar-rich variety, akin to fenite, is most common (Fig. 4c). Other clearly identifiable clasts include nepheline syenite (Fig. 4d), phonolite (Fig. 4e) and basement gneiss (Fig. 4f), reflecting the surrounding units through which the breccia was emplaced. In addition, the breccia also features many fragments of coarse-grained, magnetite-bearing, calcite carbonatite (Fig. 4g), which is mineralogically similar to the C1 carbonatite described at Songwe Hill (Broom-Fendley et al. Reference Broom-Fendley, Brady, Horstwood, Woolley, Mtegha, Wall, Dawes and Gunn2017b). Locally, the rocks are clast-poor and have a groundmass which is conspicuously K-feldspar-phyric, with the phenocrysts exhibiting a trachytic texture. Some of the feldspar is euhedral, but many grains are fragmented and broken (Fig. 4c).

Fig. 4. Field photos of breccia from around Mauze (a–g) and Nkalonje (h). (a) Typical float sample of a xenotime-rich breccia from Mantrap, showing characteristic knobbly texture and buff–pink colour. (b) Fresh surface of the same sample (a) exhibiting substantial breakdown of the primary minerals to clay. (c) Characteristic weathered surface of a mineralized breccia, with abundant clasts and K-feldspar. (d–g) Examples of different clasts, including (d) nepheline syenite, (e) phonolite, (f) gneiss and (g) carbonatite. Note the presence of a 2–3 cm alteration rim on the phonolite clasts in (e). (h) Similar breccia sampled from Nkalonje, exhibiting a clast of nepheline syenite(?), hosted in a K-feldspar phyric groundmass. K-fsp = K-feldspar; N-Sy = nepheline syenite; Phon = phonolite; carb = carbonatite.
2.2 The Nkalonje carbonatite complex
Nkalonje is located c. 10 km NW of the Songwe Hill / Mauze complex (Fig. 1b). The complex consists of several small hills that largely consist of fenitized basement with only a few dyke rocks. Most of the complex is focused on Nkalonje/Nyama Hill, which consists of a nepheline syenite plug, minor carbonatite dykes and a large round breccia unit (Garson, Reference Garson1965).
The main breccia unit at Nkalonje is c. 800 m wide and consists of trachyte, melanephelinite, metamorphic basement and K-feldspar-rich fenite fragments in a comminuted matrix. Fragments of breccia commonly contain vesicular patches of fluorite. Locally, the matrix exhibits a trachytic texture, consisting of phenocrysts of K-feldspar and accessory zircon and apatite, thought to represent recrystallized comminuted matrix (Garson, Reference Garson1965, Woolley, Reference Woolley2001; Fig. 4h).
3. Petrography of the breccias
Crystal phases and their petrographic relationships were determined on polished thin-sections at Camborne School of Mines, University of Exeter, using conventional transmitted light microscopy, CITL Mk3 and Mk5 cold-cathodoluminescence (CL) imaging equipment and a FEI Quanta 650 FEG scanning electron microscope (SEM) with energy-dispersive spectrometers (EDS).
3.1 Breccias associated with the Songwe–Mauze complex
The breccias at Mauze are fenitized xenolith-rich, porphyritic phonolites, the groundmass of which contains an assemblage of fluorite, apatite, Fe- and Mn-(hydr)oxides, HREE- and HFSE minerals. Fenitization is evident from the buff–pink colour of the rocks, their high alkali contents and an absence of quartz.
Samples from the School vents form the basis for interpreting the textures of more strongly mineralized and altered samples from Mantrap and the North vents. K-feldspar is abundant and forms large (1–25 mm), unbroken euhedral phenocrysts (Fig. 5a), with a trachytic texture in some samples. Pseudomorphs after nepheline occur as a subordinate phase and similarly form large euhedral phenocrysts. Accessories include subhedral zircon and euhedral apatite. Mafic phenocrysts are absent; however, minor Fe-(hydr)oxide-rich trapezoids may represent amphibole pseudomorphs. The groundmass is made up of microcrystalline K-feldspar, with abundant patches of Fe-/Mn-(hydr)oxides and clay minerals. Minor flow banding occurs in some fresher samples (Fig. 5b).

Fig. 5. Plane polarized light (PPL) images of breccia from Mauze. (a) Phonolite with euhedral K-feldspar (Kfs), locally altered to clay minerals, and euhedral hexagonal pseudomorphs after nepheline (Nph_psd). (b) Banding in phonolite groundmass, cross-cut by hematite-bearing vein. (c) Aligned K-feldspar and nepheline phenocrysts brecciated and cross-cut by hematite-bearing assemblage.
In all samples the primary phenocrysts are broken down to some degree, and in many cases are altered such that only a pseudomorph remains. Most K-feldspar phenocrysts are turbid and, in CL images (Fig. 6), exhibit distinct red luminescence, characteristic of fenitization (Mariano & King, Reference Mariano and King1975; Finch & Klein, Reference Finch and Klein1999; Mariano & Mariano, Reference Mariano, Mariano and Coulson2014; Elliott et al. Reference Elliott, Wall, Chakhmouradian, Siegfried, Dahlgren, Weatherley, Finch, Marks, Dowman and Deady2018; Baele et al. Reference Baele, Decrée, Rusk, Decrée and Robb2019), becoming progressively browner with higher degrees of alteration to fine-grained clay minerals. Mineralized samples are typically brecciated (Fig. 6b–c), and are clast-supported with angular to sub-angular rotated clasts (chaotic/float breccia; Woodcock & Mort, Reference Woodcock and Mort2008), and a matrix consisting of comminuted potassic fenite. Within the comminuted groundmass is an assemblage of Mn-, Fe- and Ba-bearing (hydr)oxides, such as hollandite, as well as non-luminescent low-Mg calcite, fluorite, apatite, HFSE-bearing minerals, comminuted K-feldspar, illite, quartz and a substantial amount of pore space (Fig. 5b–c). Small veins of calcite carbonatite also occur locally in a limited number of samples and can envelop fragments of fenite.

Fig. 6. Macro-scale CL images of three samples with progressively increasing HREE contents. Note the highly altered nature of the feldspar phenocrysts and groundmass in all samples. (a) T0137: note the lack of extensive brecciation, but nonetheless highly altered nature of the feldspars, presence of fluorite (Fl) and finely disseminated apatite (Ap). (b) SoS_134: note the brecciation of K-feldspar and the change in luminescence colour from red to brown, as well as the crystallization of finely disseminated violet-luminescing apatite and small grains of zircon (Zrn). (c) H0214: note the extreme brecciation, very localized occurrence of red-luminescing K-feldspar, widespread occurrence of violet–white apatite, as well as xenotime and zircon.
3.2 HREE and HFSE mineralization at the Songwe–Mauze complex
The predominant HREE and HFSE minerals in the mineralized assemblage are xenotime-(Y), Nb- and V-bearing rutile/anatase and zircon, as well as minor amounts of Th-rich monazite, thorite/huttonite, an unidentified Th–Si–P mineral and LREE fluorcarbonates. The growth of these phases is intimately associated with the breakdown and recrystallization of apatite, zircon and Fe-oxides.
Mineralization occurs in two different habits. Where present, pre-existing zircon grains have epitaxial overgrowths of isostructural xenotime-(Y) (Fig. 7a–b), as well as Nb- and Fe-bearing rutile/anatase (Fig. 7b–c). In these cases, zircon is clearly broken down, as demonstrated by the rounding of the original grain boundaries, as well as fracturing, fragmentation, embayment, local development of porosity and a darkening in BSE images (Fig. 7a–c). Xenotime overgrowths exhibit complex zoning, with porous dissolution zones and precipitation of Th-rich horizons (Fig. 7a–b). Rutile/anatase overgrowths are similarly highly porous and zoned.
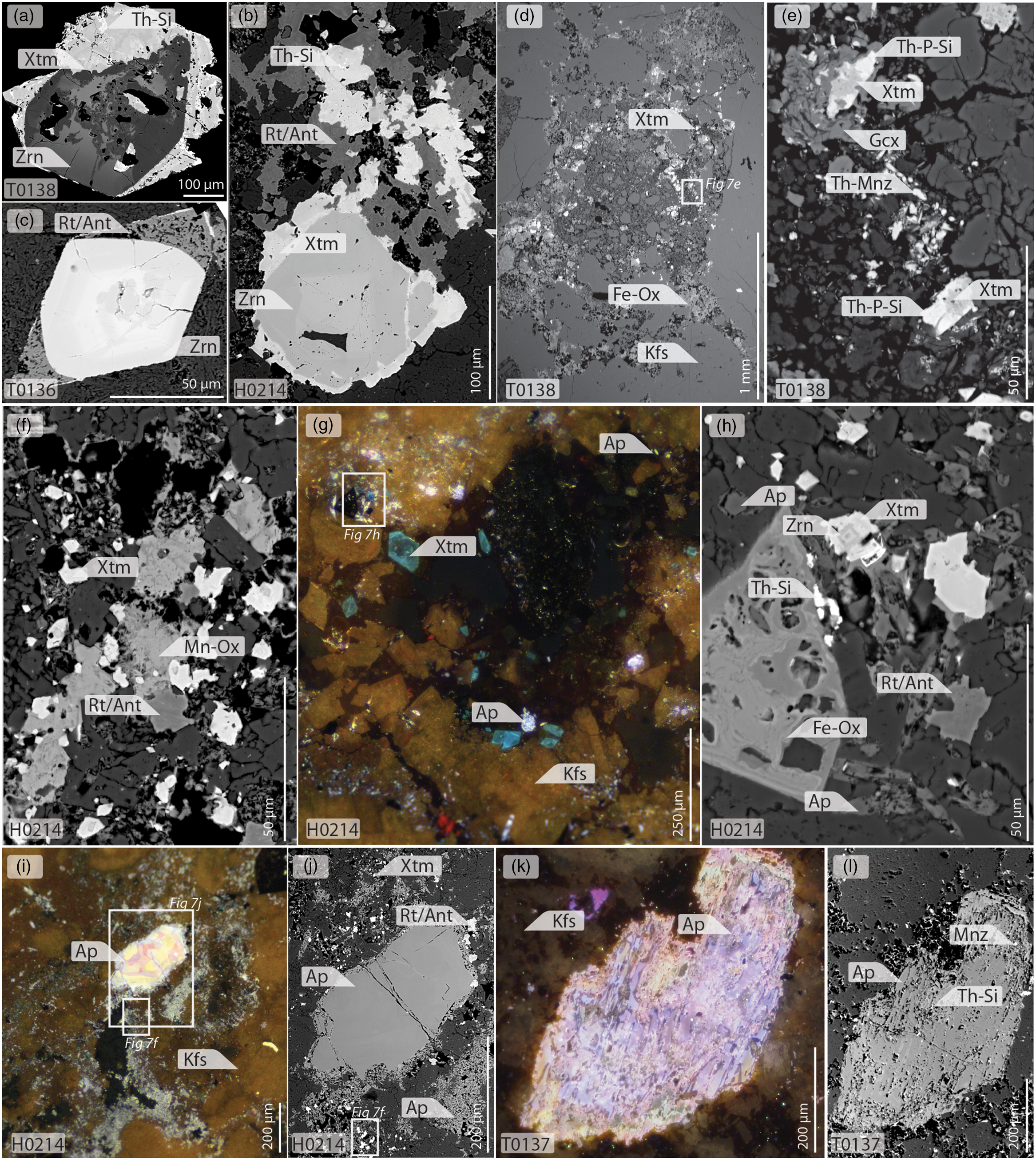
Fig. 7. BSE (a–f, h, j, l) and CL (g, i, k) images of HREE and HFSE mineralization. (a–c) Xenotime and rutile/anatase (Rt/Ant) overgrowing zircon. Note the presence of fine fractures, embayments and locally porous nature of the zircons, and the zoning and small huttonite/thorite (Th–Si) minerals in the xenotime overgrowths. (d–h) disseminated zircon, xenotime, Th-rich monazite (Th-Mnz), a REE-deficient Th–Si–P mineral (Th-P-Si), rutile/anatase and gorceixite (Gcx) in breccia. (i–l) Partially (i–j) and fully (k–l) broken-down apatite grains. Note in (i) the thin zone of partial dissolution around the edge of the large grain, and the presence of fine, violet-luminescent apatite disseminated throughout the groundmass, as well as xenotime. Note in (l) the presence of small grains of monazite and huttonite/thorite in the porous apatite.
Xenotime and rutile/anatase on pre-existing zircon grains accounts for most of the mineralization in samples with relatively low Y contents. However, in samples where Y contents are higher, these phases occur as stringers of euhedral to subhedral grains within the comminuted groundmass of the most brecciated samples (Fig. 6b–c, 7d–h). Very small (1–5 µm) euhedral zircon is disseminated throughout the mineralized zones, akin to the ‘nano-zircons’ described by Dowman et al. (Reference Dowman, Wall, Jeffries, Treloar, Carter and Rankin2017b). Commonly, such zircons form the core of xenotime-(Y) grains (Fig. 7h), which can reach up to 50 µm in size, but are most commonly c. 10–20 µm. These xenotime grains cathodoluminesce a distinct teal-blue colour (Fig. 7g). Xenotime is typically overgrown by Nb- and V-bearing anhedral rutile/anatase, which, in turn, is overgrown by Mn–Fe-(hydr)oxides (Fig. 7c, f). Locally, xenotime is also overgrown by a REE-absent, Th–Si–P phase, and is associated with the growth of euhedral Th-rich monazite and gorceixite (Fig. 7e).
Apatite occurs in all samples, in two distinct habits. Early apatite forms large, rounded grains with luminescence colours ranging from buff–pink to yellow (Fig. 6c, 7i). Early apatite is partially to completely broken down, and replaced by complex porous and, locally, skeletal violet-luminescent apatite (Fig. 7i–l); 1–10 µm huttonite/thorite and monazite grains occur within these porous horizons (Fig. 7l). In xenotime-rich samples, euhedral xenotime post-dates the breakdown of apatite (Fig. 7j).
3.3 Breccias associated with the Nkalonje complex
The field relationships and hand-specimen-scale characteristics of breccias associated with the Nkalonje complex are similar to those surrounding the Mauze nepheline syenite. The Nkalonje breccias are predominantly composed of brecciated feldspar-rich fenite, with clasts of nepheline syenite and basement gneiss. However, the matrix of these breccias is composed of quartz, fluorite, Fe oxides and weathered pseudomorphs likely to be after siderite. REE-bearing minerals are rare, and HFSE minerals are limited to small grains of zircon and rutile/anatase.
4. Whole-rock composition of the breccia rocks
Nineteen samples were analysed for major and trace element compositions (Table 1), carried out in three tranches. The first six samples were analysed by Intertek-Genalysis, Australia, by inductively coupled plasma optical emission spectrometry (ICP-OES) and mass spectrometry (ICP-MS), with powdered samples prepared as sodium peroxide fusions (see Broom-Fendley et al. Reference Broom-Fendley, Brady, Wall, Gunn and Dawes2017a for details). The second tranche of an additional four analyses was also analysed at Intertek-Genalysis, using the same instrumentation, but with samples instead prepared as Li metaborate fusions. The remaining nine analyses were undertaken at ALS Loughrea, Ireland, again by ICP-OES and ICP-MS, following technique code CCP-01. Sample powders were prepared by dissolution of Li metaborate fusions and by four-acid digestion on unfused powders, the latter technique being used for analysis of Li, Ag, As, Cd, Co, Cu, Mo, Ni, Pb, Sc, Tl and Zn. C and S were analysed using a LECO furnace. Owing to the visibly altered and, locally, weathered nature of the samples, as well as the inherent heterogeneity when analysing breccia samples, caution is warranted when interpreting the major element data, especially in respect to elements which are mobile in the weathering environment, such as Na.
Table 1. Major and trace element composition of whole-rock samples from breccias around Mauze and Nkalonje. Blank cells denote elements not analysed. Elements below the limit of detection are denoted by ‘<’

*S measured as total S, recalculated to SO3; C measured as total C, recalculated to CO2. † S measured by ICP OES, after LiBO2 fusion. Fe2O3 t = total Fe as Fe2O3
Chondrite-normalized REE distributions for unmineralized Mauze breccia samples exhibit a steep negative slope from La to Gd and then a relatively flat distribution to Lu (Fig. 8a). This distribution matches that of unaltered phonolite dykes and nepheline syenite from Mauze but with a greater REE content (Broom-Fendley, Reference Broom-Fendley2015; Chiwona et al. Reference Chiwona, Cortés, Gaulton and Manning2020). Mineralized breccia samples have a similar LREE pattern, but exhibit a steep inflection at Eu forming a convex distribution of HREE, peaking at Ho. Some samples from the School vents and from Nkalonje similarly exhibit inflection in HREE contents, although the magnitude of HREE enrichment is minor, and lacking in most samples.

Fig. 8. Chondrite-normalized (after McDonough and Sun, Reference McDonough and Sun1995) whole-rock REE distributions of (a) samples from this study, compared to nepheline syenite and phonolite from Mauze (Broom-Fendley, Reference Broom-Fendley2015 and unpub. data; Chiwona et al. Reference Chiwona, Cortés, Gaulton and Manning2020) and carbonatite from Songwe Hill (Broom-Fendley et al. Reference Broom-Fendley, Brady, Wall, Gunn and Dawes2017a); and (b) comparison samples from the Cole HFSE+HREE deposit (Andersen et al. Reference Andersen, Clark, Larson and Neill2016), Lofdal (Loye, Reference Loye2014; Namibia Rare Earths, unpub. data), and ‘high-grade’ fenite from Chilwa Island (Dowman et al. Reference Dowman, Wall, Treloar and Rankin2017a). HREE-poor samples are excluded from (b) for clarity.
In addition to HREE enrichment, several samples also exhibit high Ti, Zr, Th and/or U contents when compared to the local Songwe Hill carbonatite (Fig. 9a–e). Zirconium contents, as an example, reach up to 12 200 µg g−1 in one of the Mauze samples and over 4600 µg g−1 in a sample from Nkalonje (Table 1). Elevated Ti, Zr and Nb contents follow two trends: one where these elements correlate with increasing Y contents, and another where elevated TiO2, Zr and Nb are unrelated to Y (Fig. 9a–c). Only samples from Mantrap exhibit positive correlation between Y and Ti, Zr, and Nb. Th and U contents positively correlate with Y in all breccia suites (Fig. 9d–e).

Fig. 9. Whole-rock geochemical data from the Mauze breccias, Nkalonje and comparison data from the Songwe Hill carbonatite (Broom-Fendley et al. Reference Broom-Fendley, Brady, Wall, Gunn and Dawes2017a) and Lofdal (Loye, Reference Loye2014; Namibia Rare Earths, unpub. data). Note that comparison data from the Cole HFSE+HREE occurrence are not shown owing to their much higher Y contents. (a–e) Binary plots showing the relationship of various HFSE with Y, as a proxy for the HREE. (f) Sr against LOI (loss on ignition), demonstrating elevated Sr in samples with higher LOI. Arrows have been added to highlight the predominant trends in the Mauze (orange) and Lofdal (blue) data.
Sample T0311 contains more than 10 wt % CO2. This elevated CO2 content correlates with increased Sr and LREE, demonstrating that this sample is composed, in part, of carbonatite (Fig. 9f). Importantly, however, there is no correlation between CO2 and the HREE or HFSE contents.
5. Discussion
5.1 Evidence for carbonatite at depth
The breccias around Mauze are 1–2 km away from the Songwe Hill carbonatite (Fig. 2a), and a direct link between these bodies is not apparent. Nonetheless, the presence of calcite carbonatite xenoliths (Fig. 4g), and minor amounts of fluorite- and apatite-bearing carbonatite veins (e.g. T0311; Table 1) clearly demonstrates the small-scale presence of carbonatite at the present level of erosion, and supports the notion of further carbonatite at depth. Moreover, the composition and texture of the fenitized phonolite is similar to the equivalent fenitized phonolite at Songwe Hill (Broom-Fendley et al. Reference Broom-Fendley, Brady, Wall, Gunn and Dawes2017a). We suggest that the breccia occurrences around Mauze represent small satellite vents to the main Songwe Hill carbonatite (Fig. 10).
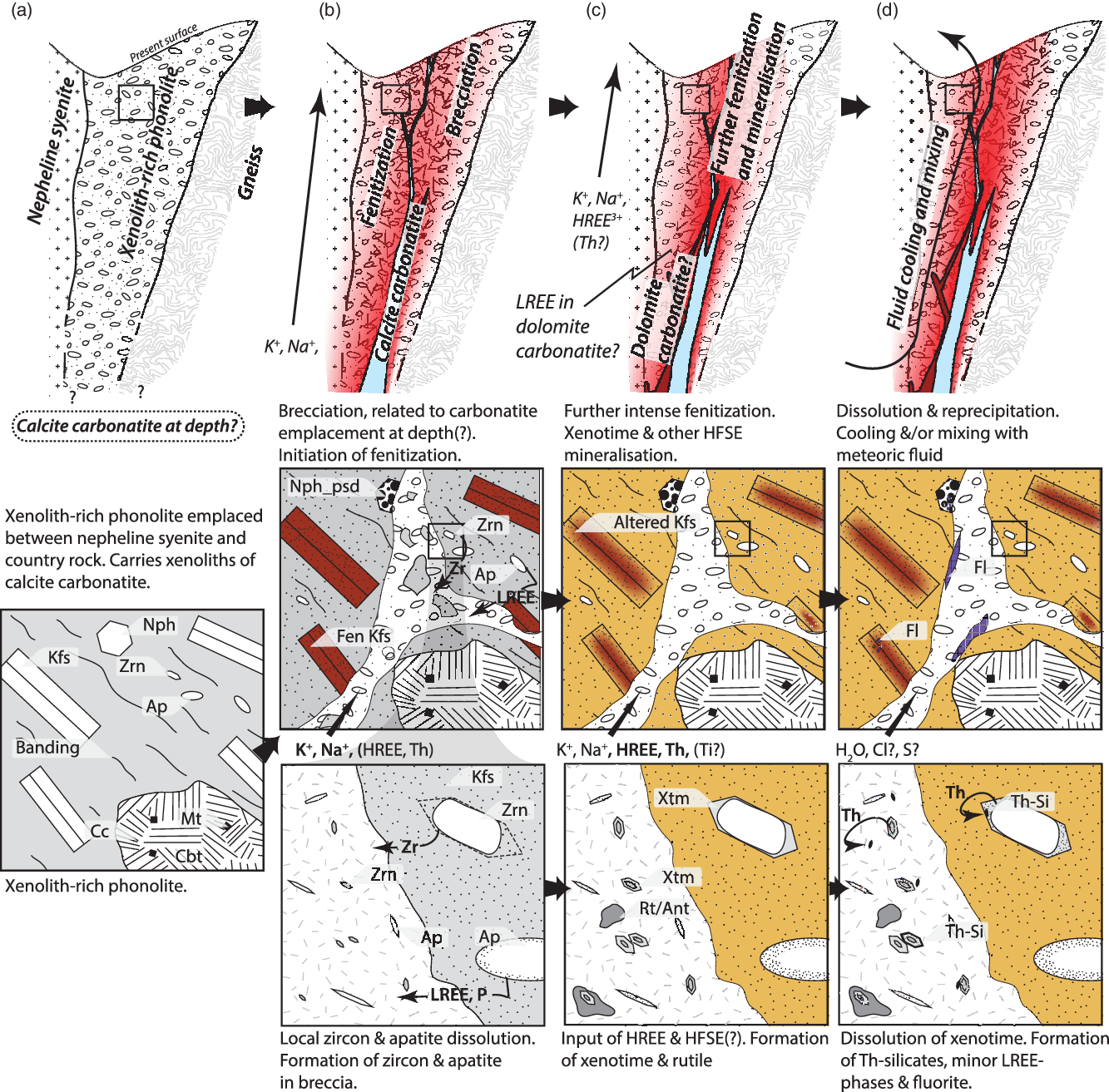
Fig. 10. Conceptual model of HREE (and HFSE) mineralization. (a) Emplacement of xenolith-rich phonolite at the boundary between nepheline syenite and country rock. (b) Brecciation and emplacement of calcite carbonatite, initial fenitization. (c) Further intense fenitization focused along breccia pipe. HREE and HFSE transported in fenitizing fluid, speculatively derived from a LREE-rich dolomite carbonatite at depth. Xenotime and rutile precipitate on pre-existing zircon and in the groundmass of the breccia. (d) Continued fluid–rock interaction results in localized dissolution and reprecipitation of xenotime, and the formation of huttonite/thorite.
5.2 Order of brecciation and mineralization
Cross-cutting textures indicate that brecciation, fenitization and crystallization of HREE minerals at Mauze occur after the initial emplacement of the xenolith-rich phonolite bodies (Fig. 10). Based on the angular nature of the clasts and the comminuted groundmass, the breccia formed by in situ rapid volume expansion (Jébrak, Reference Jébrak1997), most likely as a result of subsurface explosive release of volatiles from the proposed underlying carbonatite bodies. The result is a vertical breccia pipe which likely grades down into an underlying carbonatite body at depth. The groundmass of the breccia contains xenotime associated with late carbonate minerals, fluorite and apatite (Fig. 6a–b). These apatite- and fluorite-bearing carbonate veins are similar to veins at Songwe Hill and the adjacent fenitized breccia on Chenga Hill (Fig. 2a; Broom-Fendley et al. Reference Broom-Fendley, Brady, Wall, Gunn and Dawes2017a). Similar features include the violet-coloured apatite luminescence, presence of fluorite, xenotime, zircon, rutile/anatase and Mn- and Fe oxides, as well as the heavily altered nature of the fenite host rocks. Combined, these features further support the notion that a carbonatite-derived fluid is responsible for the HREE mineralization, and that the emplacement of such a fluid post-dates the formation of the vent rocks.
Mineral overgrowths demonstrate that the crystallization order of the REE- and HFSE-bearing minerals is consistent in each of the three mineralized fenite vents associated with the Songwe–Mauze complex. After brecciation, pre-existing zircon and apatite grains underwent dissolution, as reflected by fracturing, embayments and locally porous textures (Figs 7a–c, i–l, 10b). Simultaneously, or not long thereafter, zircon recrystallized as micron-sized euhedral grains and apatite formed needle-like and skeletal grains within the groundmass of the breccia. Partially dissolved and recrystallized micron-sized zircon both serve as a seed for isostructural growth of xenotime, which is subsequently overgrown by Nb- and V-bearing rutile and, later, Mn-, Fe- and Ba-bearing (hydr)oxides (Fig. 10c). Localized dissolution and reprecipitation of xenotime is evident from the presence of porous growth bands, formation of Th silicates and monazite, and complex zoning (Figs 7a, g, 10d).
5.3 A conceptual model for HREE mineralization in fenite breccia
A wide range of processes have been proposed to account for HREE enrichment in carbonatites. Such processes include: (1) melting of eclogitic garnet from the mantle source region during the ascent or stalled ascent of a carbonatite melt (Song et al. Reference Song, Xu, Smith, Kynicky, Huang, Wei, Zhou and Shu2016); (2) precipitation of a LREE-rich mineral, such as bastnäsite or monazite, from a carbonatite melt/fluid, thus depleting LREE in the residual melt phase and therefore relatively enriching it in HREE (Xu et al. Reference Xu, Campbell, Allen, Huang, Qi, Zhang and Zhang2007; Andersen et al. Reference Andersen, Clark, Larson and Donovan2017; Anenburg et al. Reference Anenburg, Mavrogenes, Frigo and Wall2020); and (3) redistribution of REE during late-stage hydrothermal alteration, owing to the preferential stability of REE-chloride complexes (Broom-Fendley et al. Reference Broom-Fendley, Styles, Appleton, Gunn and Wall2016a, Reference Broom-Fendley, Brady, Horstwood, Woolley, Mtegha, Wall, Dawes and Gunn2017b). All three processes, or a combination thereof, can occur at a given locality (e.g. Huanglongpu and Huayangchuan, China; Smith et al. Reference Smith, Kynicky, Xu, Song, Spratt, Jeffries, Brtnicky, Kopriva and Cangelosi2018; Cangelosi et al. Reference Cangelosi, Smith, Banks and Yardley2020a). Major source contributions from eclogitic garnet can be discounted as these would result in regional-scale HREE enrichment. While Songwe Hill does exhibit whole-rock HREE grades that are slightly elevated when compared to other REE deposits in southern Africa (Harmer & Nex, Reference Harmer and Nex2016), most carbonatites and alkali silicate rocks in the Chilwa Alkaline Province are LREE-rich.
The textural and field evidence discussed above clearly demonstrates that the HREE, Ti, Zr and Th mineralization is a hydrothermal process relating to brecciation peripheral to intrusive carbonatite bodies. As such breccias are characterized by a high initial porosity of 20–30 % and high permeability (Stripp et al. Reference Stripp, Field, Schumacher, Sparks and Cressey2006), we propose that the breccia acted as a conduit for a carbonatite-derived mineralizing fluid (Fig. 10b). Some degree of REE transport occurs in and around REE-mineralized carbonatites, and Songwe Hill is no exception (Broom-Fendley et al. Reference Broom-Fendley, Styles, Appleton, Gunn and Wall2016a, Reference Broom-Fendley, Brady, Wall, Gunn and Dawes2017a). REE can be transferred from carbonatite into the surrounding fenite aureole (Dowman et al. Reference Dowman, Wall, Treloar and Rankin2017a; Elliott et al. Reference Elliott, Wall, Chakhmouradian, Siegfried, Dahlgren, Weatherley, Finch, Marks, Dowman and Deady2018) or as small-scale (in situ) dissolution and reprecipitation of REE-bearing phases within carbonatite bodies (Broom-Fendley et al. Reference Broom-Fendley, Styles, Appleton, Gunn and Wall2016a; Benaouda et al. Reference Benaouda, Devey, Badra and Ennaciri2017; Cangelosi et al. Reference Cangelosi, Broom-Fendley, Banks, Morgan and Yardley2020b). Here, we propose that mineralization encompasses both of these mechanisms and occurs as a two-step process. Initial mineralization forms due to the transport of HREE away from a LREE-mineralized carbonatite by a fenitizing fluid. Subsequently, HREE contents are locally upgraded by later, lower-temperature, fluid-mediated, dissolution–reprecipitation processes.
5.3.1 HREE transport and precipitation
The initial mineralization step proposed here presumes (1) prior precipitation of LREE-rich minerals in a cooling, crystallizing carbonatite at depth, or (2) a direct relationship between the breccia vents at Mauze and the LREE-mineralized Songwe Hill carbonatite. Neither interpretation can be proved on the basis of the evidence presented, but each may be justified considering the evolution of the Songwe Hill carbonatite and the recent experimental results of Anenburg et al. (Reference Anenburg, Mavrogenes, Frigo and Wall2020). These authors demonstrate that alkali–LREE–carbonate minerals precipitate from a Na–K–carbonate±chloride±sulphate-brine phase which itself represents the residuum from crystallization of a carbonatitic melt (Prokopyev et al. Reference Prokopyev, Borisenko, Borovikov and Pavlova2016). During crystallization of the carbonatitic melt, the HREE are incompatible in the crystallizing assemblage so are retained in the residual Na- and K-rich fluid phase (Anenburg et al. Reference Anenburg, Mavrogenes, Frigo and Wall2020). Similar alkali-rich fluids have been proposed as the origin of fenite alteration aureoles adjacent to carbonatites, as supported by the presence of alkali carbonate daughter minerals in fluid inclusions (Bühn & Rankin, Reference Bühn and Rankin1999; Williams-Jones & Palmer, Reference Williams-Jones and Palmer2002; Dowman et al. Reference Dowman, Wall, Treloar and Rankin2017a; Prokopyev et al. Reference Prokopyev, Kozlov, Fomina, Doroshkevich and Dyomkin2020).
The field and mineralogical evidence presented here is consistent with the model for HREE mineralization proposed by Anenburg et al. (Reference Anenburg, Mavrogenes, Frigo and Wall2020). Here, a carbonatite intrusion undergoes extensive differentiation and small amounts of HREE become concentrated in the residual liquid phase. This liquid is localized into the overlying permeable structure of vent breccias. Alteration of the breccia rocks to a fenite assemblage coincides with the precipitation of the HREE minerals from this liquid. Xenotime evidently forms as a euhedral phase during, or not long after, the initial brecciation (Fig. 7f–h). Apatite, too, is introduced during this initial fenitization, but is considerably altered by subsequent hydrothermal fluids (Fig. 7i–j). Thus, we propose that the HREE-rich mineralization in fenite breccia is the result of prior crystallization of LREE minerals, either from an unexposed carbonatite body intruded into the fenite breccia vent at depth, or from the nearby Songwe Hill carbonatite (Fig. 10).
5.3.2 Local dissolution–reprecipitation
In addition to early xenotime precipitation, several features indicate that a subsequent hydrothermal fluid mediates dissolution and reprecipitation of REE- and HFSE-bearing minerals in the fenite breccias. Fluid-mediated dissolution–reprecipitation is common in carbonatites whereby cooling hydrothermal fluids back-react with a parent mineral, forming new phases from the trace element contents of the parent phase (Putnis, Reference Putnis2009). Such a process is common in phases that contain REE as minor components, such as apatite and calcite (Broom-Fendley et al. Reference Broom-Fendley, Styles, Appleton, Gunn and Wall2016a, b; Cangelosi et al. Reference Cangelosi, Smith, Banks and Yardley2020a; Ying et al. Reference Ying, Chen, Simonetti, Jiang and Zhao2020). It is evident that dissolution–reprecipitation has occurred in zircon, apatite and xenotime at several stages of the Mauze fenite breccias. In all cases, the principal evidence is the volume reduction in the parent phase, as indicated by porosity and fractures (e.g. zircon: Fig. 7a; xenotime: Fig. 7e; and apatite: Fig. 7l). Additionally, the product phase crystallizes in close proximity to the parent, such as the isostructural overgrowths of xenotime on zircon (Fig. 7a) and the local formation of huttonite/thorite on xenotime (Fig. 7e). Where dissolution–reprecipitation has occurred in xenotime and zircon, the HREE- and Th phases are retained close to the original mineral (Fig. 7a, e), while LREE minerals form new euhedral phases, in close proximity (Fig. 7e). The substantially larger size and higher abundance of the xenotime grains indicates that zircon breakdown is not the sole source of the HREE in these rocks.
While the proximity of the product phases indicates that REE transport is probably not substantial, the difference in Th content and LREE/HREE contents of the reprecipitated minerals indicates that the altering fluid was capable of fractionating the REE. Experimental work demonstrates that both chloride and fluoride are capable of fractionating the REE, under acidic conditions, owing to the different stabilities of the LREE and HREE chloride and fluoride complexes (Migdisov et al. Reference Migdisov, Williams-Jones and Wagner2009). However, the low solubility of REE fluorides means the capacity for F to transport the REE is limited (Migdisov et al. Reference Migdisov, Williams-Jones, Brugger and Caporuscio2016). We speculate that after crystallization, xenotime is altered by a Cl-bearing hydrothermal fluid and this fluid redistributes the LREE to a new LREE phase, while retaining the HREE and Th in close proximity to the parent mineral (Fig. 10d). Such a mechanism was proposed by Williams-Jones et al. (Reference Williams-Jones, Wollenberg, Bodeving, Simandl and Neetz2015) to have led to the HREE mineralization at Lofdal, although here we only consider the transport and fractionation to occur on a scale of a few metres.
5.4 Comparing the Chilwa Province fenite breccias with other carbonatite-associated HREE and HFSE mineralization
The vent breccias described here are not as HREE-rich as peralkaline-related HREE deposits (Dostal, Reference Dostal2017) or unconformity-related xenotime mineralization (Nazari-Dehkordi et al. Reference Nazari-Dehkordi, Spandler, Oliver and Wilson2018), but they far exceed the HREE contents of most examples of carbonatite and fenite. As a basis for comparison, Figures 8b and 9a–e include data from the Cole occurrence (Andersen et al. Reference Andersen, Clark, Larson and Neill2016) and the Lofdal deposit (Do Cabo, Reference Do Cabo2013; Loye, Reference Loye2014; Namibia Rare Earths, unpub. data), as well as high-grade fenite breccia from Chilwa Island (Dowman et al. Reference Dowman, Wall, Treloar and Rankin2017a).
All of these examples are located adjacent to intrusive carbonatite complexes and are HREE-enriched when compared to these adjacent complexes and to typical carbonatite compositions. All are associated with brecciated rocks which have been metasomatized to increase their alkali content: those at the Cole occurrence are composed predominantly of K-feldspar (Andersen et al. Reference Andersen, Clark, Larson and Neill2016), while at Lofdal (Area 4), mineralization is associated with albitite (HS Swinden & P Siegfried, unpub. technical report, 2011). In both occurrences xenotime occurs as a mixture of disseminated fine grains and overgrowths on older zircon cores, and is associated with fluorite.
The REE pattern of mineralized Mauze vent breccias broadly matches the REE distribution of all three comparison examples (Fig. 8b), with an inflection to elevated HREE contents occurring between Nd–Eu, depending on the LREE content of the sample. Yttrium contents (as a proxy for total HREE) are much lower in the Mauze vent breccias than those at the Cole occurrence and also typically lower than samples from Lofdal (Figs 8b, 9a). The HREE contents of the most HREE-rich fenite sample from Chilwa Island correspond to the least-mineralized Mauze breccia samples (Fig. 8b). The Mauze breccias share elevated HFSE contents, similar to those reported from the Lofdal Deposit and the Cole occurrence. Samples from Lofdal define a similar correlation between Y, Ti and U to the mineralized Mauze samples (Fig. 9a, e). Thorium contents also increase with Y in Lofdal samples, but not as steeply as those from Mauze breccia. While Zr and Nb contents from Lofdal are correlated in the data presented here (Loye, Reference Loye2014; Fig. 9b–c), this relationship is not apparent when considering all analyses of drillcore from Lofdal (Namibia Rare Earths, unpub. data).
In addition to the few carbonatite localities where xenotime-(Y) or HREE enrichment has been documented, there are also examples of carbonatite-related alteration with elevated Ti, Zr, Th or Nb contents. These include: the Christy deposit of the Magnet Cove complex, USA (Flohr, Reference Flohr1994); Salpeterkop and Goudini, South Africa (Verwoerd et al. Reference Verwoerd, Viljoen and Chevallier1995; Verwoerd, Reference Verwoerd2008a, b) and Gross Brukkaros, Namibia (Werner & Cook, Reference Werner and Cook2001). Many of these examples share textural and geochemical features with the mineralization at Mauze. Of particular note are the Christy deposit and Salpeterkop. Both of these localities host Ti in brookite, with grades sufficiently high at the Christy deposit to warrant extraction on a small scale between 1934 and 1944 (Flohr, Reference Flohr1994). At the Christy deposit, Ti minerals predominantly occur in highly altered alkali-rich country rock associated with clay-rich dykes with abundant relict K-feldspar. At Salpeterkop, mineralization is hosted in highly altered breccia, associated with abundant K-feldspar and trachyte (Verwoerd et al. Reference Verwoerd, Viljoen and Chevallier1995). These associated rocks bear some similarity to the deposits discussed above, and of particular note is that the Christy clay-rich dykes and the Salpeterkop breccia are both locally HREE-enriched.
While the REE grades in fenite are typically low (Fig. 8b; Elliott et al. Reference Elliott, Wall, Chakhmouradian, Siegfried, Dahlgren, Weatherley, Finch, Marks, Dowman and Deady2018), REE mineralization can still occur in this rock up to several kilometres from the parent carbonatite intrusion (Dowman et al. Reference Dowman, Wall, Treloar and Rankin2017a). REE mineralization in fenite is limited to small vein networks and pockets, which can also include monazite, xenotime, bastnäsite, parisite, ancylite, as well as Th and Nb minerals. In particular, xenotime and Th-rich minerals can occur in the highest-grade fenites found at the Chilwa Island complex, Malawi (Dowman et al. Reference Dowman, Wall, Treloar and Rankin2017a). While they contain far lower HREE contents than the samples described here, these xenotime-bearing micro-mineral assemblages bear strong textural and petrographic similarities to the mineralization at Mauze. The host rocks are near-identical and the late-stage nature of the mineralization is highly similar. Dowman et al. (Reference Dowman, Wall, Jeffries, Treloar, Carter and Rankin2017b) also note that zircon in the most intensely fenitized rocks exhibits dissolution textures and forms the nucleus for later REE mineral precipitation, features we also report from the Mauze breccias (Fig. 7a).
Based on the similarities to the relatively HREE-rich micro-mineral assemblages present in the Chilwa Island fenite, we suggest that some degree of HREE transport occurs in all fenite assemblages – particularly high-grade K-rich fenite. The substantially higher HREE contents of the fenitized vent rocks at Mauze, however, may be caused by the focusing of fluids through highly permeable breccia, which would lead to a greater degree of HREE input than the comparatively lower-grade fenites studied by Dowman et al. (Reference Dowman, Wall, Treloar and Rankin2017a). We propose that similar HREE mineralization at Lofdal and the Cole HFSE/HREE deposit represents extreme focusing of a similar residual fluid, and that all examples of HREE mineralization in fenites peripheral to carbonatites share a similar causal link.
5.5 HREE-rich fenite breccias as an exploration indicator for LREE mineralization
The mineralization reported here is not of sufficiently high grade or large enough to be economic in its own right. Moreover, the HREE enrichment is intimately associated with an increase in Th, the processing of which provides environmental and social challenges (Findeiß & Schäffer, Reference Findeiß and Schäffer2017). Nonetheless, enrichment of HREE and Th in fenite breccias serves as a record of fluid expulsion from a REE-rich carbonatite, and may indicate the presence of LREE mineralization in carbonatite at depth. The Chilwa Alkaline Province features at least 13 breccia vents similar to those investigated in this study. Many of these are associated with carbonatite (Garson, Reference Garson1965), but have not yet been studied with respect to their REE mineralization potential. Minor carbonate veins, and a proximity to other carbonatite complexes, suggest that these vents may be related to the emplacement of carbonatite intrusions below the present level of erosion. In particular, if HREE mineralization occurs due to the prior formation of LREE minerals, then a HREE-enriched vent may be an exploration indicator for a LREE deposit at depth (Dowman et al. Reference Dowman, Wall, Treloar and Rankin2017a; Elliott et al. Reference Elliott, Wall, Chakhmouradian, Siegfried, Dahlgren, Weatherley, Finch, Marks, Dowman and Deady2018). Importantly, the link between HREE and Th contents (Figs 2b, 9d) demonstrates that radiometric surveys may be used to locate near-surface enrichment of HREE and, therefore, any potentially associated LREE mineralization.
Vent breccias at the Nkalonje complex and the small Malagani vent, to the northwest of Songwe (Fig. 1), were investigated in this study as a comparison to mineralized vents around Mauze. These share superficial similarities to the mineralized vents (Fig. 4h), but both laboratory whole-rock analyses and field analyses using a portable X-ray fluorescence instrument do not indicate HREE mineralization. HFSE minerals are limited to localized occurrences of anatase/rutile. Carbonatite is scarce at Nkalonje, with only minor REE-rich dykes, and is absent at Malagani, so we speculate that the lack of HFSE and HREE minerals in their fenites reflects the low REE content of their parent carbonatites.
6. Conclusions
-
HREE mineralization occurs in fenitized xenolith-rich phonolite breccia, peripheral to the Songwe Hill carbonatite and adjacent to the Mauze nepheline syenite, Malawi.
-
Fenitization and brecciation post-date the emplacement of phonolite and minor carbonatite, with possible further carbonatite present at depth.
-
Mineralization predominantly consists of xenotime, with zircon, anatase/rutile and minor huttonite/thorite hosting other HFSE. Zircon formed both from the mineralizing fluid as well as providing the seed for further epitactic xenotime growth.
-
The mineralization at Mauze is texturally and compositionally similar to higher-grade HREE-bearing rocks at the Cole occurrence (USA) and the Lofdal deposit (Namibia), as well as minor HREE enrichment in fenite at the Chilwa Island carbonatite (Malawi).
-
The formation of HREE-rich fenite may reflect the passage of liquids derived from an intrusive carbonatite body that have undergone extensive fractional crystallization. Experimental evidence suggests that during this crystallization the LREE cargo of the carbonatite is deposited as mineralization at depth. HREE-rich fenite may therefore represent an exploration indicator for LREE deposits in the associated intrusive carbonatite body.
-
We suggest that HREE transport and fractionation occurs in most cases of fenitization, but enrichment to the concentrations occurring in the Mauze breccia requires the focusing of fenite fluid through pre-existing structures.
-
The link between HREE and Th contents demonstrates that radiometric surveys may be used to locate near-surface enrichment of HREE in fenite breccias.
Acknowledgements
We are grateful to James Mtegha, Ansel Zabula, Innocencia Nkumila and Chikondi Mcheka (Mkango Resources) for their contributions to Figure 2b and constructive field discussions, and to Enoch Chitsulo for assisting with fieldwork – including important snake and buffalo bean identification. Scott Swinden kindly provided unpublished data on the Lofdal deposit and comments on an early version of the manuscript. We also thank two anonymous reviewers for their comments. This work was funded by a Natural Environment Research Council (NERC) Industrial Innovation Fellowship to S.B-F. (NE/R013403/1), supported by contributions from Mkango Resources, the NERC SoS RARE consortium (NE/M011429/1) and the EU H2020 HiTech AlkCarb program grant agreement no. 689909.
Conflicts of interest
None.













