1. Introduction
Evolutionary innovations on an unprecedented scale are observed in Ediacaran biotas in regard to morphological disparity and ecological adaptations. Macroscopic organisms are recorded as soft-bodied impressions, carbonaceous compressions and mineralized and organically preserved bodies in various environmental settings, ranging from shallow marine to offshore and deep basinal (Narbonne et al. Reference Narbonne, Laflamme, Trusler, Darlymple and Greentree2014; Wan et al. Reference Wan, Yuan, Chen, Guan, Pang, Tang and Xiao2016; Warren et al. Reference Warren, Quaglio, Simões, Gaucher, Riccomini, Poiré, Freitas, Boggiani and Sial2017; Wood et al. Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019). The macrobiota displays many novel morphological traits that are difficult to relate to modern organisms. However, sponges, placozoans, cnidarians, lophophorates and probable bilaterians have been interpreted to be among them (Fedonkin et al. Reference Fedonkin, Gehling, Grey, Narbonne and Vickers-Rich2007; Xiao & Laflamme, Reference Xiao and Laflamme2009; Wood et al. Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019). In the case of microscopic fossils, there are some that are recognizable and similar, in terms of general body plan and individual characters, to those known in the Palaeozoic and among extant microorganisms (Grey, Reference Grey2005; Moczydłowska, Reference Moczydłowska2010, Reference Agić, Moczydłowska and Canfield2016). Phenotypic eukaryotic characteristics, including functional morphological and reproductive structures, can be used to link Ediacaran microfossils with modern phyla and classes.
Resistant, organic-walled microfossils recovered from the Pertatataka Formation in Australia were first recognized as Ediacaran in age (Zang & Walter, Reference Zang and Walter1989, Reference Zang and Walter1992) and were regarded as typifying the diversity and morphological complexity of the Ediacaran Period (635–541 Ma; Condon et al. Reference Condon, Zhu, Bowring, Wang, Yang and Jin2005; Grey, Reference Grey2005). Their remarkably high diversity is shown by variously ornamented vesicles with larger dimensions than Phanerozoic microfossils, resulting in describing over 100 form-species globally (Grey, Reference Grey2005; Liu & Moczydłowska, Reference Liu and Moczydłowska2019). It was apparent that the same type of Ediacaran microfossils occurred abundantly, had various modes of preservation (organically preserved and diagenetically permineralized by silification and phosphatization) and had been previously recorded in China and Siberia, but these microfossils were attributed to regional Sinian and Vendian chronostratigraphic units, respectively (Timofeev, Reference Timofeev1969; Yin & Li, Reference Yin and Li1978; Zhang, Reference Zhang1981; Pyatiletov & Rudavskaya, Reference Pyatiletov, Rudavskaya, Sokolov and Ivanovskij1985; Yin, Reference Yin1985). Ediacaran microfossils have now been extensively studied in the Doushantuo Formation in South China (Zhang et al. Reference Zhang, Yin, Xiao and Knoll1998; Liu et al. Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; Xiao et al. Reference Xiao, Zhou, Liu, Wang and Yuan2014; Liu & Moczydłowska, Reference Liu and Moczydłowska2019) as well as in several successions in Siberia (Moczydłowska et al. Reference Moczydłowska, Vidal and Rudavskaya1993; Sergeev et al. Reference Sergeev, Knoll and Vorobeva2011; Moczydłowska & Nagovitsin, Reference Moczydłowska and Nagovitsin2012), Baltica (Veis et al. Reference Veis, Vorobeva and Golubkova2006; Vorobeva et al. Reference Vorobeva, Sergeev and Knoll2009), India (Shukla & Tiwari, Reference Shukla and Tiwari2014; Prasad & Asher, Reference Prasad and Asher2016) and Mongolia (Anderson et al. Reference Anderson, Macdonald, Jones, McMahon and Briggs2017, Reference Anderson, McMahon, Macdonald, Jones and Briggs2019).
Ediacaran microfossils were considered to be largely phytoplanktonic and algal in affinity. This interpretation of organically preserved microfossils as representing algal cysts was based on their morphological comparisons with extant taxa, vesicle wall biochemical resistance and was supported by case studies of the wall ultrastructure in certain species (Zang & Walter, Reference Zang and Walter1989, Reference Zang and Walter1992; Arouri et al. Reference Arouri, Greenwood and Walter1999, Reference Arouri, Greenwood and Walter2000; Grey, Reference Grey2005; Moczydłowska, Reference Moczydłowska2005, Reference Agić, Moczydłowska and Canfield2016; Willman & Moczydłowska, Reference Willman and Moczydłowska2007; Moczydłowska & Willman, Reference Moczydłowska and Willman2009; Moczydłowska et al. Reference Moczydłowska, Landing, Zang and Palacios2011).
Some phosphatized microfossils with dividing cells preserved inside the vesicle and recovered from the Doushantuo Formation in the Weng’an locality in South China were interpreted as animal eggs and embryos (Xiao et al. Reference Xiao, Zhang and Knoll1998; Xiao & Knoll, Reference Xiao and Knoll2000), such as Tianzhushania and its putative developmental stages: Megasphaera, Parapandorina and Megaclonophycus; and Spiralicellula and Caveasphaera (Xiao et al. Reference Xiao, Zhang and Knoll1998; Xiao & Knoll, Reference Xiao and Knoll2000; C Yin et al. Reference Yin, Bengtson and Yue2004; Xiao et al. Reference Xiao, Hagadorn, Zhou and Yuan2007a; L Yin et al. Reference Yin, Zhu, Knoll, Yuan, Zhang and Hu2007). The Tianzhushania plexus was alternatively interpreted with a broader holozoan (animals and protists related to animals; Torruella et al. Reference Torruella, de Mendoza, Grau-Bové, Antó, Chaplin, del Camplo, Eme, Pérez-Cordón, Whipps, Nichols, Paley, Roger, Sitjà-Bobadilla, Donachie and Ruiz-Trillo2015) affinity (Huldtgren et al. Reference Huldtgren, Cunningham, Yin, Stampanoni, Marone, Donoghue and Bengtson2011). However, the algal affinity remains possible (Butterfield, Reference Butterfield2011). Zhang and Pratt (Reference Zhang and Pratt2014) argued on the basis of inferred reproductive life cycle for a chlorophyte algal origin for Spiralicellula and Helicoforamina, which they interpreted as the same biological taxon. Note, however, that Helicoforamina can also be treated as a distinct taxon instead of being a developmental stage and was recently suggested to have holozoan affinity (Yin et al. Reference Yin, Sun, Liu, Zhu and Donoghue2020). A holozoan affinity has also been specifically proposed for Cavasphaera (Yin et al. Reference Yin, Vargas, Cunningham, Bengtson, Zhu, Marone and Donoghue2019).
Certain organically preserved microfossils previously inferred to be algal cysts, such as Appendisphaera, Alicesphaeridium and Gyalosphaeridium (Grey, Reference Grey2005; Moczydłowska, Reference Moczydłowska2005), were also assumed to represent animal diapause cysts (Yin et al. Reference Yin, Zhu, Knoll, Yuan, Zhang and Hu2007; Cohen et al. Reference Cohen, Knoll and Kodner2009). This affiliation was not substantiated by the presence of animal reproductive characters other than surficial cyst morphology.
The animal cyst and embryo hypothesis of the Ediacaran microfossils has been both supported and critically scrutinized, suggesting alternative bacterial, holozoan and green algal affinities for these concerned taxa. A bacterial origin (Bailey et al. Reference Bailey, Joye, Kalanetra, Flood and Corsetti2007 a, b) has been abandoned because neither ornamented nor spinose envelopes like those in Tianzhushania or Megasphaera exist in bacteria (Xiao et al. Reference Xiao, Zhou and Yuan2007 b), nor the differentiated nuclei observed in Megasphaera (although referred to as Tianzhushania) and Spiralicellula (Huldtgren et al. Reference Huldtgren, Cunningham, Yin, Stampanoni, Marone, Donoghue and Bengtson2011; Donoghue et al. Reference Donoghue, Cunningham, Dong, Bengtson and Wanninger2015; see also Cunningham et al. Reference Cunningham, Thomas, Bengtson, Marone, Stampanoni, Turner, Bailey, Raff, Raff and Donoghue2012) and unnamed embryos (Hagadorn et al. Reference Hagadorn, Xiao, Donoghue, Bengtson, Gostling, Pawlowska, Raff, Raff, Turner, Yin, Zhou, Yuan, McFeely, Stampanoni and Nealson2006). Among alternative interpretations of the Ediacaran microfossil affinities (Xue et al. Reference Xue, Tang, Yu and Zhou1995; Hagadorn et al. Reference Hagadorn, Xiao, Donoghue, Bengtson, Gostling, Pawlowska, Raff, Raff, Turner, Yin, Zhou, Yuan, McFeely, Stampanoni and Nealson2006; Butterfield, Reference Butterfield2011; Huldtgren et al. Reference Huldtgren, Cunningham, Yin, Stampanoni, Marone, Donoghue and Bengtson2011; Yin et al. Reference Yin, Zhu, Tafforeau, Chen, Liu and Li2013, Reference Yin, Vargas, Cunningham, Bengtson, Zhu, Marone and Donoghue2019; Zhang & Pratt, Reference Zhang and Pratt2014; Donoghue et al. Reference Donoghue, Cunningham, Dong, Bengtson and Wanninger2015; Moczydłowska, Reference Agić, Moczydłowska and Canfield2016; Cunningham et al. Reference Cunningham, Vargas, Yin, Bengtson and Donoghue2017) it is acknowledged that these microfossils are cysts – but of what origin: algae, holozoans or metazoans?
We describe new specimens with internal bodies that represent single and multiple dividing cells in seven studied species of Appendisphaera, Mengeosphaera, Tanarium, Urasphaera and Tianzhushania, as well as those known in some other Ediacaran morphotypes still left without interpretation which add critical evidence and are significant for unravelling the biological affinities of the microbiota. We document, for the first time, cell division in statu nascendi of forming cleavage and in late developmental stages that are diagnostic for recognizing algal cysts vs animal diapause cysts among microfossils, including the putative animal embryo Tianzhushania spinosa. We provide examples of extant algal taxa that are phenotypically analogous to these microfossils and have the same biochemical resistance properties to decay (as a function of cyst wall composition) and we analyse various lines of evidence in the studied species to support an algal biological affinity and to question previous interpretations.
2. Materials, preservation and methods
Newly recorded organic-walled microfossils derive from chert nodules in the dolostone and mudstone of the Ediacaran Doushantuo Formation (635–551 Ma; Condon et al. Reference Condon, Zhu, Bowring, Wang, Yang and Jin2005), which was studied in several geological successions in the Yangtze Gorges area, South China (Fig. 1; Supplementary Figs S1–S5 in the Supplementary Material available online at https://doi.org/10.1017/S0016756820001405; see Liu & Moczydłowska, Reference Liu and Moczydłowska2019 for geological details). The Doushantuo Formation is a c. 220 m thick succession of siliciclastic and carbonate rocks referred to four informal members (I–IV) and deposited in shallow marine shelf to slope depositional environments on the Yangtze Platform (Jiang et al. Reference Jiang, Shi, Zhang, Wang and Xiao2011). The lowermost member, I, a cap dolostone, is un-fossiliferous, and the uppermost member, IV (or the Miaohe member), has not yet yielded microfossils but contains macroscopic carbonaceous compression fossils (Steiner, Reference Steiner1994; Xiao et al. Reference Xiao, Yuan, Steiner and Knoll2002, Reference Xiao, Kowalewski, Sheng, Dong and Laflamme2010; Ye et al. Reference Ye, Tong, An, Hu, Tian, Guan and Xiao2019). Chert samples for our study were collected from the dolostone and mudstone in members II and III exposed at the Liuhuiwan, northern Xiaofenghe, Wangfenggang, Niuping and Dinshuiyan sections (Supplementary Figs S1–S5 in the Supplementary Material available online at https://doi.org/10.1017/S0016756820001405), and their stratigraphic logs and the occurrence of microfossils were reported in detail by Liu et al. (Reference Liu, Xiao, Yin, Chen, Zhou and Li2014) and Liu & Moczydłowska (Reference Liu and Moczydłowska2019). These strata are of early-middle Ediacaran age (Fig. 1). From a taxonomically rich assemblage of microfossils, we selected for the present study only those species and specimens preserving internal bodies and dividing cells within the vesicle cavity that are indicative of biological affinities of microfossils.
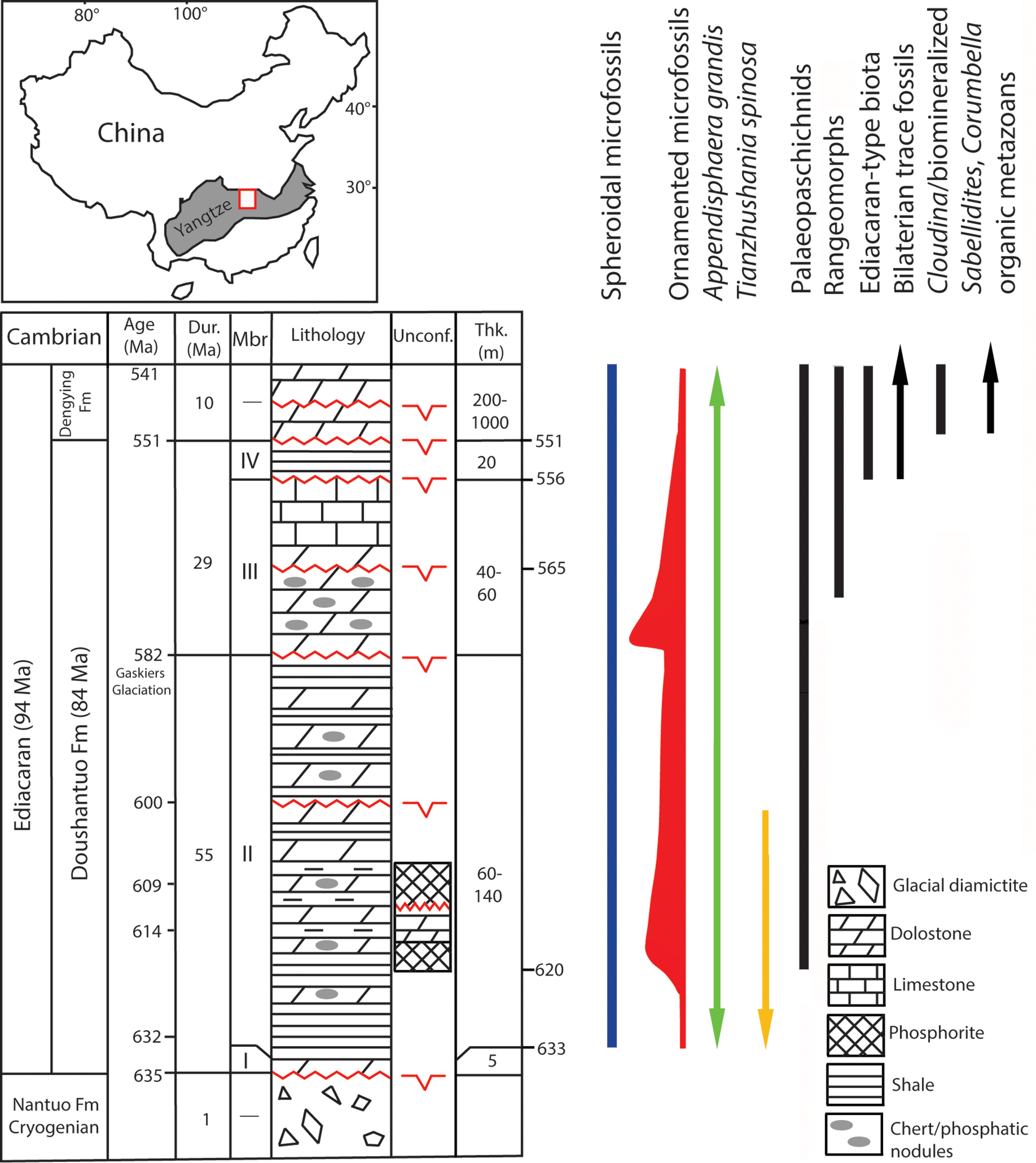
Fig. 1. Generalized Ediacaran geological succession in South China showing the stratigraphic ranges of selected microfossils and characteristic macroscopic groups from other occurrences, with all ranges as globally recognized. The ornamented microfossils’ relative diversity is marked by range line thicknesses. The location of Yangtze Gorges study area is marked by the square in the shaded area of the Yangtze Block. The uppermost range of microfossils is not recorded in China but in terminal Ediacaran in Mongolia (Anderson et al. Reference Anderson, Macdonald, Jones, McMahon and Briggs2017). Macrofossil distribution is according to Narbonne et al. (Reference Narbonne, Xiao, Shields, Gradstein, Ogg, Schmitz and Ogg2012) and Kolesnikov et al. (Reference Kolesnikov, Rogov, Bykova, Danelian, Clausen, Maslov and Grazhdankin2018) for palaeopascichnids, and Matthews et al. (Reference Matthews, Liu, Yang, McIlroy, Levell and Condon2020) for the age of rangeomorphs at 574 Ma. Cryogenian, Ediacaran, Cambrian refer to Period/System. Fm, Formation; Dur, Duration; Mbr, Member; Unconf., Unconformity; Thk, Thickness. Geological succession in South China is compiled from sources cited in text and revised in Liu & Moczydłowska (Reference Liu and Moczydłowska2019). The unconformities are recognized by Wang et al. (Reference Wang, Erdtmann, Chen and Mao1998), Zhang et al. (Reference Zhang, Jiang and Han2008), Lu et al. (Reference Lu, Zhu and Zhao2012), Zhu et al. (Reference Zhu, Lu, Zhang, Zhao, Li, Aihua, Zhao and Zhao2013), Liu & Moczydłowska (Reference Liu and Moczydłowska2019).
Microfossils were examined in petrographic thin-sections of chert nodules that are c. 50 μm thick under transmitted- and plane-polarized light microscope (LM). Chert nodules were cut parallel and perpendicular to the dolostone and mudstone bedding plane. The state of preservation induced by diagenetic permineralization is exceptional, demonstrating details of vesicle morphology and internal cells. The microfossils are ornamented by processes and contain a single internal body to multiple internal cells with uniquely preserved cleaving cells in seven studied taxa (Figs 2 and 3 further below). These are Appendisphaera grandis Moczydłowska et al. Reference Moczydłowska, Vidal and Rudavskaya1993, emend. Moczydłowska, Reference Moczydłowska2005, A. tabifica Moczydłowska et al. Reference Moczydłowska, Vidal and Rudavskaya1993, Mengeosphaera bellula Liu et al. Reference Liu, Xiao, Yin, Chen, Zhou and Li2014, M. sp., Tanarium paucispinosum Grey, Reference Grey2005, Urasphaera fungiformis Liu et al. Reference Liu, Xiao, Yin, Chen, Zhou and Li2014, and Tianzhushania spinosa Yin & Li, Reference Yin and Li1978, emend. Yin, Reference Yin, Liu, Zhao, Xing and Ding1988 (Yin & Liu, Reference Yin, Liu, Zhao, Xing and Ding1988). Internal structures within vesicles occur in only one or a few specimens per species. The majority of specimens for each species show only empty vesicle cavities, but both preservation types co-occur in the same samples. This is a common preservation bias seen in the studied and some other Ediacaran species (cf. Liu et al. Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; Xiao et al. Reference Xiao, Zhou, Liu, Wang and Yuan2014; Moczydłowska, Reference Agić, Moczydłowska and Canfield2016).
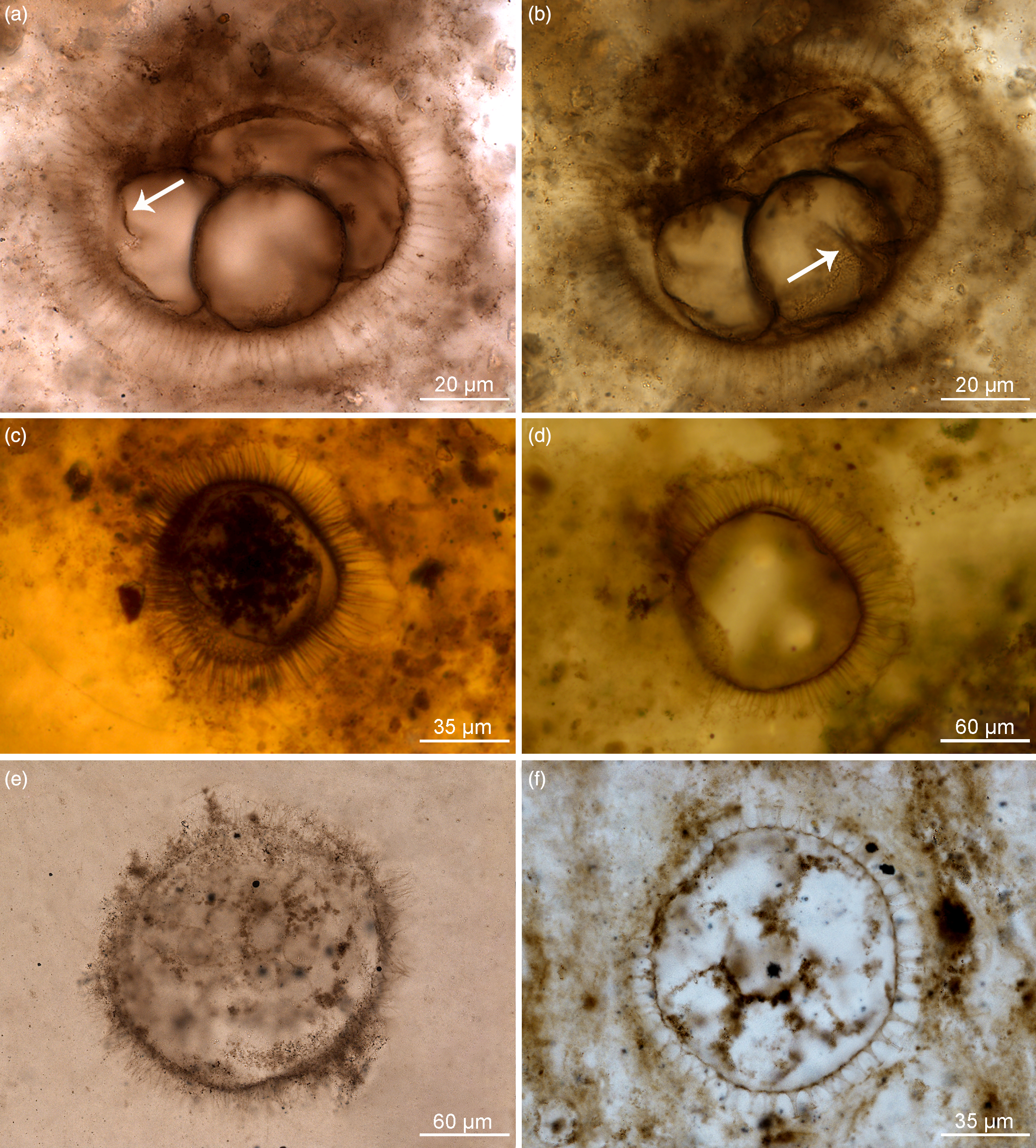
Fig. 2. Organic-walled microfossils containing internal body and dividing cells within acanthomorphic reproductive cysts. (a–d) Appendisphaera grandis. (a, b) Specimen at different focus levels showing four cells at the initial cleavage stage within vesicle cavity and initial wall furrow of cells (white arrow); IGCAGS-LHW145, LHW6.6-7(M44/3), depth 6.6 m at Liuhuiwan section. (c) Spheroidal endocyst containing multiple cells preserved within the cyst cavity; IGCAGS-D2XFH371, XFH0946-1-57, depth 113.0 m at northern Xiaofenghe section. (d) Vesicle with emptied cavity diagenetically replaced by silica; IGCAGS-D2XFH674, XFH0946-1-182(X51/2), depth 113.0 m at northern Xiaofenghe section. (e) Appendisphaera tabifica containing multiple spheroidal cells; IGCAGS-WF109, WFG48.3-1(M33), depth 48.3 m at Wangfenggang section. (f) Urasphaera fungiformis showing several cells within the cyst cavity; IGCAGS-NPIII111, NPIII-16(M14), depth 185.0 m at Niuping section. All are transmitted-light micrographs.

Fig. 3. Organic-walled microfossils containing internal body and multiple cells within cyst cavity. (a, b) Mengeosphaera bellula. (a) Specimen preserving a single internal body, the endocyst; IGCAGS-DSY286, DSY17-16(L25), depth 17.0 m at Dishuiyan section. (b) Specimen containing multiple cells embraced by membranous endocyst within the cyst cavity; IGCAGS-DSY067, DSY8-13(O38), depth 8.0 m at Dishuiyan section. (c) Mengeosphaera sp., at a stage of four-cells division; IGCAGS-DSY165, DSY11.5-14(G39), depth 11.5 m at Dishuiyan section. (d) Tanarium paucispinosum showing multiple-celled cluster within the cyst cavity; IGCAGS-LHW058, LHW-0.35-2(D47), depth –0.35 m at Liuhuiwan section. (e, f) Tianzhushania spinosa preserved at the stage of a few internal cells in (e) and with multiple spheroidal cells in (f). (e) Specimen IGCAGS-XFH653, XFH0946-1-174 (T49/4), depth 113.0 m at northern Xiaofenghe section. (f) Specimen IGCAGS-XFH598, XFH0946-1-162 (D23/4), depth 113.0 m at northern Xiaofenghe section. All are transmitted-light micrographs.
All genera, with the exception of Mengeosphaera and Tianzhushania, are also known from Siberia, Australia and Baltica (Grey, Reference Grey2005; Moczydłowska, Reference Moczydłowska2005; Vorobeva et al. Reference Vorobeva, Sergeev and Knoll2009), where they are organically preserved, extracted from the host sediment by acid maceration (standard palynological treatment with hydrofluoric and hydrochloric acids) and thus proven to be decay- and acid-resistant. The same properties may be foreseen for Mengeosphaera and Tianzhushania judging from their robustness (observed here) and three-dimensional (3D) preservation (when both silicified and phosphatized). The studied microfossils consist of refractory biopolymers in their walls. In chert preservation studied here, they are encrusted by amorphous silica, which also impregnates vesicle cavities and internal bodies (seen as white material in photomicrographs) due to diagenetic permineralization. The carbonaceous material comprising the vesicles of microfossils is revealed and characterized in the same taxa, i.e. Appendisphaera, Mengeosphaera and Tianzhushania, and from the same successions studied by laser Raman spectroscopy and transmitted- and plane-polarized light microscopy, as are the diagenetic processes leading to their silicification (Shang et al. Reference Shang, Moczydłowska, Liu and Liu2018, Note error in double printing of Tianzhushania spinosa in figs 6 and 7, instead of Appendisphaera tenuis in fig. 6; see Supplementary Fig. S6 in the Supplementary Material available online at https://doi.org/10.1017/S0016756820001405, whereas Tianzhushania spinosa is correct in fig. 7).
Microfossils were photographed by digital camera, the images were not enhanced digitally and the colours are genuine as seen in LM. The specimens’ cross-sections show vesicle outline, processes, and individual cells within the vesicle cavity and their spatial arrangement in clusters. The palaeontological material is stored in the collections of the Institute of Geology, Chinese Academy of Geological Sciences, Beijing, China. The illustrated specimens are designated by the prefix IGCAGS followed by the sample number and specimen position by England Finder graticules in thin-section orientated with its label to the left side.
3. Results
In palaeontological descriptions, the morphological characteristics of studied species focus on phenotypic features and specifically the newly observed internal cells and their geometry in various ontogenetic stages that are of paramount importance for unravelling the biological affinities of the microbiota. The species identification is in concert with their diagnoses, and no unusual features are observed other than the preservation of single internal bodies and multiple dividing cells inside the vesicles. These internal structures, large single spheroidal bodies and multiple individual identical cells in clusters are interpreted as representing reproductive, developmental stages of cyst containing endocyst and offspring cells, respectively, based on comparison with other Ediacaran microfossils of similar morphotypes and extant algal species (Moczydłowska, Reference Agić, Moczydłowska and Canfield2016). The characteristic ornamentation of process-bearing (acanthomorphic) vesicles is used to recognize form-taxa on the basis of their shape, size and configuration on the vesicle surface.
3.a. Appendisphaera
The form-genus Appendisphaera Moczydłowska et al. Reference Moczydłowska, Vidal and Rudavskaya1993, emend. Moczydłowska, Reference Moczydłowska2005, with type species A. grandis, is characterized by lavishly ornamented vesicles bearing cylindrical and hollow processes freely communicating with vesicle cavity (Moczydłowska, Reference Moczydłowska2005). Thirteen species are recognized, identified by their disparate process morphology, of which many are cosmopolitan in distribution and known from Siberia, Baltica, Australia, China and Mongolia palaeocontinents (Grey, Reference Grey2005; Moczydłowska, Reference Moczydłowska2005; Vorobeva et al. Reference Vorobeva, Sergeev and Knoll2009; Anderson et al. Reference Anderson, Macdonald, Jones, McMahon and Briggs2017, Reference Anderson, McMahon, Macdonald, Jones and Briggs2019; Liu and Moczydłowska, Reference Liu and Moczydłowska2019). The vesicle wall is organically preserved in three dimensions and robust enough to be extracted by chemical treatment from shale sediment.
Appendisphaera grandis is very regular in shape due to the abundance of homomorphic long processes that are symmetrically arranged (Fig. 2a–d) and it may possess a circular excystment opening (pylome) depending on its developmental stage (Moczydłowska, Reference Moczydłowska2005). Processes are slim cylindrical in shape with slightly widened bases and tapering distally to sharp-pointed tips (Fig. 2a–d). The vesicle comprises a few to multiple internal bodies, which are the dividing cells (Fig. 2a–c). The internal cells are here observed in this species for the first time, and a superbly preserved single specimen contains four spheroidal cells in the vesicle cavity, which show wall furrows at the initial cleavage stage (Fig. 2a, b). These equal-sized cells are arranged in a planar tetrad and are attached to one another along portions of their walls. The wall furrows are invaginated across half of the cell surface (Fig. 2b). The vesicle of this specimen is 70–78 μm in diameter while the process length is 14–16 μm and the individual internal cells are 33–35 μm in diameter (Fig. 2a, b). Another specimen of a similar vesicle size includes multiple cells that are 9–11 μm in diameter and are surrounded by a membranous sack (interpreted to be an endocyst), which is 46–62 μm in diameter (Fig. 2c). In several other specimens, there are multiple and much smaller spheroidal cells clustered together, but not compressed, and enclosed within the endocyst, which is clearly detached from the vesicle’s inner wall (Fig. 2c). Most specimens are preserved with empty vesicle cavities (n = 70; Fig. 2d). Total vesicle diameter range of the species is 50–812 μm.
A.grandis is a cosmopolitan species, and its first appearance datum (FAD) globally is established at 9.4 m above the base of the Doushantuo Formation in the Wangfenggang section (Liu & Moczydłowska, Reference Liu and Moczydłowska2019; Supplementary Fig. S2 in the Supplementary Material available online at https://doi.org/10.1017/S0016756820001405). The age of this stratigraphic level is slightly younger than that of the Doushantuo Formation’s lower boundary at c. 635 Ma and is estimated to c. 633 Ma. The FAD of A. grandis makes it among the earliest Ediacaran microfossils globally and substantially precedes the Ediacara-type impression macrofossils that appeared at c. 571 Ma or 574 Ma (Pu et al. Reference Pu, Bowring, Ramezani, Myrow, Raub, Landing, Mille, Hodgin and Macdonald2016; Matthews et al. Reference Matthews, Liu, Yang, McIlroy, Levell and Condon2020, respectively; Fig. 1). This species is contemporaneous with Tianzhushania spinosa, which is recorded at the 6.8 m level above the Doushantuo Formation base in the correlative Chenjiayuanzi section (Liu & Moczydłowska, Reference Liu and Moczydłowska2019). A. grandis stratigraphically ranges throughout most of the Doushantuo Formation in China and the entire Ediacaran System, as it was documented in Mongolia in the uppermost Ediacaran (Anderson et al. Reference Anderson, Macdonald, Jones, McMahon and Briggs2017, Reference Anderson, McMahon, Macdonald, Jones and Briggs2019; Fig. 1).
Appendisphaera tabifica (Fig. 2e) is diagnosed by short thin processes that coalesce together (Moczydłowska et al. Reference Moczydłowska, Vidal and Rudavskaya1993; Moczydłowska Reference Moczydłowska2005). The illustrated specimen’s diameter is 185 μm and the process length is 20–27 μm. This specimen contains multiple internal cells that although fading due to degradation, are clearly spheroidal and closely arranged. These cells are 23–25 μm in diameter and form a dense cluster. In another species, A. tenuis from the Doushantuo Formation in the Songlin area of Guizhou Province studied by Shang et al. (Reference Shang, Liu and Moczydłowska2019, fig. 5d), better-preserved internal cells, ten or more seen in thin-section, are recorded. These cells are identical spheroidal and clustered but not aligned in any pattern,
In all these Appendisphaera species, the vesicle cross-sections show tightly packed clusters of cells without any free cavity and the cells are spheroidal, of the same size and without any sign of shape differentiation or layer arrangement (Fig. 2c, e).
3.b. Urasphaera
Another species with a body plan of an acanthomorphic vesicle is Urasphaera fungiformis, which has conical processes with broad bases and shield-like tips, hollow inside and freely communicating with the vesicle cavity (Moczydłowska & Nagovitsin, Reference Moczydłowska and Nagovitsin2012). In a single specimen, several spheroidal cells are tightly packed within the vesicle cavity (Fig. 2f) and these seven cells visible in cross-section are 27–30 μm in diameter, whereas the entire vesicle diameter is 96 μm and the process length is 12 μm. A few other specimens preserved with empty vesicle cavity have diameter 181–250 μm and processes 22–68 μm (n = 3).
3.c. Mengeosphaera
Mengeosphaera bellula bears biform processes with conical bases and long apical spines that are hollow and freely communicate with the vesicle cavity (Liu et al. Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; Fig. 3a, b). Two specimens preserve internal bodies. One has a single, large and opaque internal body that is defined by its own membranous wall (endocyst) within the vesicle cavity (Fig. 3a), and another contains multiple spheroidal and tightly packed cells inside the endocyst (Fig. 3b). The endocyst occupies nearly the entire vesicle cavity, and its wall is detached from the vesicle wall. The equal-sized multiple cells seen in the vesicle cross-section form a dense cluster without any cavity. Both the opaque internal body and that containing multiple cells are organically preserved, as is the vesicle wall and processes, but a small part of the vesicle cavity and the spaces between processes are replaced by diagenetic silica (white material in photomicrographs). The specimens’ vesicle diameters are 60–64 μm and process length is 17–19 μm. The single endocyst diameter in one specimen is 48 μm (Fig. 3a), while in the other the endocyst with multiple cells is 56–58 μm in diameter. The multiple individual cells enclosed within this endocyst are 10–12 μm in diameter (Fig. 3b). Very abundant specimens of this species show empty cavities or occasionally preserved endocyst with disintegrated remnants of internal cells (cf. Liu et al. Reference Liu, Xiao, Yin, Chen, Zhou and Li2014, fig. 53: 8–9). The species total vesicle diameter ranges from 50 to 90 μm and the process length from 14 to 19 μm. Some other species of Mengeosphaera (see Liu et al. Reference Liu, Xiao, Yin, Chen, Zhou and Li2014) preserve dense clusters of multiple cells that are enclosed by an endocyst wall inside the vesicle cavity.
An undetermined species of Mengeosphaera, Mengeosphaera sp., is represented in this material by a specimen with four cells within the vesicle cavity, which are observed to have a planar tetrad geometry (Fig. 3c). The cells are well-defined by organic walls, but their cavities are impregnated by silica, as is the area surrounding the entire vesicle. The vesicle diameter is 50 μm and process length 15–18 μm (n = 1). The cells are 23 × 30 μm in diameter.
3.d. Tanarium
The vesicle of Tanarium paucispinosum bears a few conical processes communicating with the vesicle cavity (Grey, Reference Grey2005). We report a single specimen containing multiple, small spheroidal and closely clustered cells within the vesicle cavity (Fig. 3d) among 11 observed specimens. The individual cells are opaque and organically preserved, equal in size, very well-defined and not compressed against one another. The cells occupy most of the vesicle cavity, and its remaining part is impregnated by silica. The cluster of cells (over 50) seen in the vesicle cross-section is dense (Fig. 3d), but a small portion is degraded and replaced by silica enclosing a clotted organic matter, which is a taphonomic feature. The studied Tanarium paucispinosum vesicle diameter is 165–184 μm, process length 46 μm and the individual cells are 12–16 μm in diameter (Fig. 3d). In other specimens, the vesicle diameter ranges from 83 to 198 μm and the process length from 24 to 88 μm (n = 11).
Tanarium is a cosmopolitan form-genus and the most taxonomically diverse of the Ediacaran microfossils (18 species), showing a wide range of vesicle diameters, 32–356 μm (Liu & Moczydłowska, Reference Liu and Moczydłowska2019).
3.e. Tianzhushania
The form-genus Tianzhushania Yin & Li, Reference Yin and Li1978, emend. C Yin, Reference Yin, Liu, Zhao, Xing and Ding1988, emend. L Yin et al. Reference Yin, Zhou and Yuan2008, has large, 350–980 μm diameter vesicles bearing hollow cylindrical processes which penetrate the multilamellate layer surrounding the vesicle and support the external membrane (Yin & Liu, Reference Yin, Liu, Zhao, Xing and Ding1988; Yin et al. Reference Yin, Zhou and Yuan2008). Although not diagnosed, in various described species and other genera that were recognized as junior synonyms of Tianzhushania there are a few to numerous cells preserved within the vesicle (Xiao & Knoll, Reference Xiao and Knoll2000; C Yin et al. Reference Yin, Bengtson and Yue2004; Xiao et al. Reference Xiao, Zhou and Yuan2007 b; L Yin et al. Reference Yin, Zhu, Knoll, Yuan, Zhang and Hu2007, Reference Yin, Zhou and Yuan2008).
In this study, the type species, T. spinosa (Yin & Li, Reference Yin and Li1978) emend. Yin, 1988 (Yin & Liu, 1988; Yin et al. Reference Yin, Zhou and Yuan2008), is represented by specimens with several to multiple internal cells that are hemispherical to polygonal (in a few cells stage) or small spheroidal (in multiple cells stage) and enclosed by an internal membrane with a smooth surface within the vesicle cavity (Fig. 3e–f). In one specimen, the multiple small spheroidal cells of equal size are tightly packed in the cluster, as is seen in the vesicle cross-section consisting of c. 230 cells (Fig. 3f). This vesicle diameter is c. 700 μm and individual cells are 37 μm in diameter (n = 1; Fig. 3f), so the entire volume of the vesicle cavity likely comprised a few thousand cells.
4. Interpretation of studied species
In all described species, the internal multiple (four to thousands of) spheroidal cells of the same sizes and tightly arranged are interpreted as dividing cells inside the endocyst within the acanthomorphic cyst. A single large internal body defined by the membranous wall and occupying the entire cavity of the vesicle in Mengeosphaera bellula is interpreted as an endocyst containing zygote before undergoing division (Fig. 3a), or containing multiple dividing cells inside (Fig. 3b), as also in Appendisphaera grandis (Fig. 2c). A single, opaque in appearance endocyst represents an early developmental stage. The endocyst may not be preserved due to taphonomy or may be destroyed during the development of multiple offspring cells in mature cysts as seen in Appendisphaera tabifica (Fig. 2e), Urasphaera fungiformis (Fig. 2f), Mengeosphaera sp. (Fig. 3c), Tanarium paucispinosum (Fig. 3d) and the late stage of Tianzhushania spinosa (Fig. 3f). In all studied species, the vesicles bearing processes of various shapes, sizes and distribution, and additionally external membranes in T. spinosa, are interpreted to be reproductive cysts containing endocysts and offspring cells.
In the genus Tanarium, three other species than described T. paucispinosum, i.e. T. tuberosum, T. conoideum and T. digitiforme, were previously reported to contain a single internal body (Xiao et al. Reference Xiao, Zhou, Liu, Wang and Yuan2014; Moczydłowska, Reference Agić, Moczydłowska and Canfield2016) and were interpreted to represent an endocyst within an algal zygotic cyst (Moczydłowska, Reference Agić, Moczydłowska and Canfield2016). They show developmental stages in the complex life cycle of Tanarium. The present record of multiple cells within the vesicle cavity in T. paucispinosum supports this interpretation by documenting the more matured ontogenetic stage with a dense cluster of cells without any cavity.
Our specimen of Tianzhushania spinosa with a large number of identically sized spheroidal cells within the vesicle cavity (Fig. 3f, that would account a few thousand cells in 3D reconstruction) represents multicellular stage and demonstrates a lack of any space inside the cell cluster or cell differentiation and orientation into layers or poles. Several specimens of T. spinosa with preserved internal cells as observed here were previously reported in thin-sections from the Doushantuo Formation of the Weng’an area, Guizhou Province, and the Yichang area, Hubei Province, South China (C. Yin et al. Reference Yin, Bengtson and Yue2004; L. Yin et al. Reference Yin, Zhu, Knoll, Yuan, Zhang and Hu2007, Reference Yin, Zhou and Yuan2008, respectively). The species T. conferta Yin et al. Reference Yin, Zhou and Yuan2008, synonymous with T. spinosa (Xiao et al. Reference Xiao, Zhou, Liu, Wang and Yuan2014), contains specimens with hundreds of spheroidal cells in their vesicle cavity (Yin et al. Reference Yin, Zhou and Yuan2008) and represents a late developmental stage. Two specimens illustrated in thin-sections from the Doushantuo Formation of the Yichang area by Yin et al. (Reference Yin, Zhou and Yuan2008, pl. I, figs 11, 13) preserved multiple internal cells, which are partly taphonomically disintegrated, and a vesicle cavity which is partly diagenetically replaced by phosphate and silica. Despite this taphonomic alteration, it appears that identical cells occupied the vesicle cavity and the cells’ cluster lacks any free space as in our complete specimen (Fig. 3f). The vesicle of Tianzhushania spinosa is a cyst containing the membranous, smooth-walled endocyst within its cavity and the offspring cells. Based on the present observations and evaluating previous interpretations of Tianzhushania as metazoan or holozoan, we infer alternative affinity for this taxon (see Section 6.c).
5. Biological affinities
The dividing cells inside the cyst-like vesicle, their shape, size and spatial arrangement, have been the primary features in considering possible affinities of microfossils in previous studies and herein. However, we equally emphasize in conjunction with these features the cyst morphology, complexity and wall biochemical properties. In the search for the biological affinity of the studied microfossils, we analysed their phenotypic morphology of cysts and reproductive characters in combination with biochemical properties of the vesicle wall and their palaeoecology.
5.a. Palaeoecology
The Ediacaran microfossils occur in various facies in shallow to offshore platform and slope settings, which represent holomarine environments (Grey, Reference Grey2005; Jiang et al. Reference Jiang, Shi, Zhang, Wang and Xiao2011; Moczydłowska & Nagovitsin, Reference Moczydłowska and Nagovitsin2012; Anderson et al. Reference Anderson, Macdonald, Jones, McMahon and Briggs2017), and many are cosmopolitan. Such distribution is typical of extant phytoplankton (algae and bacteria) that may be passively dispersed globally by ocean gyres and currents over a short time of a few thousand years (Reynolds, Reference Reynolds2006). Wide geographic distribution is also known among zooplankton (Lipps, Reference Lipps1993; Garrison, Reference Garrison1999) but their cyst morphology is dissimilar to the studied microfossils (compare Porter, Reference Porter, Xiao and Kaufman2006; Bosak et al. Reference Bosak, Macdonald, Lahr and Matys2011; Morais et al. Reference Morais, Fairchild, Lahr, Rudnitzki, Schopf, Garcia, Kudryavtsev and Romero2017). Studied microfossil species are cosmopolitan (with the exception of T. spinosa so far as is known) and facies-independent which is consistent with the phytoplankton (see also Grey, Reference Grey2005; Moczydłowska, Reference Moczydłowska2005; Liu & Moczydłowska, Reference Liu and Moczydłowska2019).
5.b. Wall biochemical properties
Various species of Appendisphaera, Urasphaera and Tanarium, including those studied herein, have been previously extracted from the sedimentary rocks and preserved as 3D and robust vesicles comprising organic matter with biochemical decay resistance, a property evidenced by organic matter survival through hundreds of millions of years of geologic history, and its negative reaction to HF acid upon extraction (Moczydłowska et al. Reference Moczydłowska, Vidal and Rudavskaya1993; Grey, Reference Grey2005; Moczydłowska, Reference Moczydłowska2005, Reference Agić, Moczydłowska and Canfield2016; Vorobeva et al. Reference Vorobeva, Sergeev and Knoll2009; Moczydłowska & Nagovitsin, Reference Moczydłowska and Nagovitsin2012). Species of Tianzhushania and Mengeosphaera have not yet been extracted from the sedimentary rocks as organically preserved microfossils, but their 3D preservation shown by circular outlines of vesicles that survived the early diagenesis and permineralization by silica and phosphate without collapse as observed in thin-sections (Liu et al. Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; Xiao et al. Reference Xiao, Zhou, Liu, Wang and Yuan2014; Shang et al. Reference Shang, Moczydłowska, Liu and Liu2018; Liu & Moczydłowska, Reference Liu and Moczydłowska2019) indicates mechanical and chemical resistance. Although the fossil biopolymers are usually transformed to more recalcitrant components during diagenesis and their chemical composition may not be original, even fossil molecules (biomarkers) and traces of biopolymers could be detected in primary composition without full fossil structures in favourable conditions (Briggs & Summons, Reference Briggs and Summons2014). Specific conditions, such as aluminosilicate and kaolinite mineral coating, may stabilize organic matter and facilitate preservation of organic fossils (Anderson et al. Reference Anderson, Tosca, Cinque, Frogley, Lekkas, Akey, Hughes, Bergmann, Knoll and Briggs2020). These previously studied (Moczydłowska et al. Reference Moczydłowska, Vidal and Rudavskaya1993; Grey, Reference Grey2005; Moczydłowska, Reference Moczydłowska2005, Reference Agić, Moczydłowska and Canfield2016; Moczydłowska & Nagovitsin, Reference Moczydłowska and Nagovitsin2012; Liu et al. Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; Xiao et al. Reference Xiao, Zhou, Liu, Wang and Yuan2014; Shang et al. Reference Shang, Moczydłowska, Liu and Liu2018, Reference Shang, Liu and Moczydłowska2019; Liu & Moczydłowska, Reference Liu and Moczydłowska2019) and present microfossils derive from sediments that have undergone mild diagenesis and low thermal maturity, suggesting limited potential for organic biopolymer change over time. Consequently, the original organism biopolymers likely had similar decay resistance to those presently comprising the microfossils. Very few microfossil taxa have been studied as to their geochemical composition but, in general, phytoplankton microfossils are decay-resistant due to their cyst wall properties and therefore are abundantly preserved (Evitt, Reference Evitt1985; Colbath & Grenfell, Reference Colbath and Grenfell1995; Kokinos et al. Reference Kokinos, Eglinton, Goni Mam Boon, Martoglio and Anderson1998; Arouri et al. Reference Arouri, Greenwood and Walter2000; Marshall et al. Reference Marshall, Javaux, Knoll and Walter2005; Briggs & Summons, Reference Briggs and Summons2014). Ediacaran Tanarium conoideum examined by micro-Fourier transform infrared (FTIR) spectroscopy showed spectra that are consistent with those obtained from algaenans isolated from extant chlorophyte and eustigmatophyte microalgae (Marshall et al. Reference Marshall, Javaux, Knoll and Walter2005). The studied Tanarium species preserved not only resistant cyst wall but also internal cells.
The wall resistance properties of the studied microfossils are known among the algaenan, mannan, sporopollenin, cellulose, cutan and chitin groups of biopolymers and among these, the first three groups are known in algal cysts (Atkinson et al. Reference Atkinson, Gunning and John1972; Evitt, Reference Evitt1985; Derenne et al. Reference Derenne, Largeau, Berkalo, Rousseau, Wilhelm and Hatcher1992 a, b, Reference Derenne, Largeau and Berkalo1996; Gelin et al. Reference Gelin, Volkman, Largeau, Derenne, Sinninghe Damsté and De Leeuw1999; Allard & Templier, Reference Allard and Templier2000; Hagen et al. Reference Hagen, Siegmund and Braune2002; Damiani et al. Reference Damiani, Leonardi, Pieroni and Cáceres2006; De Leeuw & Largeau, Reference De Leeuw, Largeau, Engel and Macko2006; De Leeuw et al. Reference De Leeuw, Largeau, Versteegh and Van Bergen2006). Sporopollenin, cellulose and cutan are synthesized by plants (Evitt, Reference Evitt1985; Buchanan et al. Reference Buchanan, Gruissem and Jones2000), which originated from algae that acquired chloroplasts from their ancestral cyanobacteria (Delwiche, Reference Delwiche1999; Raven et al. Reference Raven, Evert and Eichhorn2005; Keeling, Reference Keeling2010; Adl et al. Reference Adl, Bass, Lane, Lukes, Schoch, Smirnov, Agatha, Berney, Brown, Burki, Cárdenas, Cepicka, Chistyakova, del Campo, Dunthorn, Edvardsen, Eglit, Guillou, Hampl, Heiss, Hoppenrath, James, Karnkowska, Karpov, Kim, Kolisko, Kudryavtsev, Lahr, Lara, Le Gall, Lynn, Mann, Massana, Mitchell, Morrow, Soo Park, Pawlowski, Powell, Richter, Rueckert, Shadwick, Shimano, Spiegel, Torruella, Youssef, Zlatogursky and Zhang2019), and cellulose is synthesized by certain cyanobacteria (Römling & Galperin, Reference Römling and Galperin2015). All these biopolymers are produced by photosynthesizing organisms. Chitin is polymerized by rhizaria, fungi, protistan holozoans and animals (Webster & Weber, Reference Webster and Weber2007; Gupta, Reference Gupta2011; Taylor et al. Reference Taylor, Krings and Taylor2015; Torruella et al. Reference Torruella, de Mendoza, Grau-Bové, Antó, Chaplin, del Camplo, Eme, Pérez-Cordón, Whipps, Nichols, Paley, Roger, Sitjà-Bobadilla, Donachie and Ruiz-Trillo2015; Loron et al. Reference Loron, Francois, Rainbird, Turner, Borensztajn and Javaux2019). The resistant compounds were recognized as chitin in fungal microfossils at c. 1.0–0.9 Ga (Loron et al. Reference Loron, Francois, Rainbird, Turner, Borensztajn and Javaux2019), but these microfossils have no morphologic comparison to those studied here. Chitin commonly occurs in animal integuments, but chitin is also found in the egg cysts of only a few known taxa of derived phyla among the invertebrates (nematods, tardigrades, and arthropods including crustaceans and insects; Scholtz & Wolff, Reference Scholtz, Wolff, Minelli, Boxhall and Fusco2013). The fossil record indicates that these invertebrate groups evolved in the Cambrian and insects in the Devonian (Maas & Waloszek, Reference Maas and Waloszek2001; Engel & Grimaldi, Reference Engel and Grimaldi2004; Erwin & Valentine, Reference Erwin and Valentine2013). Based on molecular clock analysis, the origin of crown group animals is suggested to occur in the Tonian–Cryogenian interval at c. 833–650 Ma, yet a precise timeline of animal evolution cannot be currently obtained (dos Reis et al. Reference Dos Reis, Thawornwattana, Angelis, Telford, Donoghue and Yang2015). This estimate is approximate and the minimum age is not much older than the fossil records for the emergence of major animal phyla in the terminal Ediacaran and Cambrian (Sperling et al. Reference Sperling, Pisani, Peterson, Vickers-Rich and Komarower2007; Budd, Reference Budd2008). The above-considered phyla likely evolved in this transitional interval. Conversely, the Ordovician metazoan egg cases of ‘chitinozoans’ are not made of chitin (Jacob et al. Reference Jacob, Paris, Monod, Miller, Tang, George and Beny2007). Thus, among photosynthesizing clades in the Ediacaran time (Moczydłowska, Reference Moczydłowska2008b, Reference Agić, Moczydłowska and Canfield2016; Butterfield, Reference Butterfield2015), cyanobacteria and algae are the most likely to have produced resistant cyst walls. Although filamentous cyanobacteria produce heterocysts and akinetes with thick walls that are preservable and resistant, they lack ornamentation. The algal cysts are the best candidates because their morphology is characteristic and recognizable among the microfossils studied.
The biochemical synthesis pathway of decay-resistant, refractory biopolymers in the algal cyst wall is thought to be a shared ancestral (symplesiomorphic) character of phylogenetic lineages of basal chlorophytes and derived streptophytes leading to plants (Raven et al. Reference Raven, Evert and Eichhorn2005; Falkowski & Raven, Reference Falkowski and Raven2007; O’Kelly, Reference O’Kelly, Falkowski and Knoll2007; Baldauf, Reference Baldauf2008; Turmel et al. Reference Turmel, Brouard, Gognon, Otis and Lemieux2008; Burki et al. Reference Burki, Roger, Brown and Simpson2020). Cellulose, the most abundant biopolymer on Earth, is exceptionally resistant and is produced by plant cellulose synthase complexes; these enzyme complexes have a cyanobacterial origin and have been genetically inherited from cyanobacterial ancestors that became chloroplasts in algae and then in plants (Römling & Galperin, Reference Römling and Galperin2015). Enzymes that bind specific compounds during bacterial photosynthesis are present in all photosynthesizing organisms (David & Alm, Reference David and Alm2011); these organisms include some bacteria, algae and embryophytes (Raven et al. Reference Raven, Evert and Eichhorn2005). Among green algae, the phenotypic cyst characters are expressed in various clades of chlorophytes and streptophytes (the group Chloroplastida; Adl et al. Reference Adl, Bass, Lane, Lukes, Schoch, Smirnov, Agatha, Berney, Brown, Burki, Cárdenas, Cepicka, Chistyakova, del Campo, Dunthorn, Edvardsen, Eglit, Guillou, Hampl, Heiss, Hoppenrath, James, Karnkowska, Karpov, Kim, Kolisko, Kudryavtsev, Lahr, Lara, Le Gall, Lynn, Mann, Massana, Mitchell, Morrow, Soo Park, Pawlowski, Powell, Richter, Rueckert, Shadwick, Shimano, Spiegel, Torruella, Youssef, Zlatogursky and Zhang2019; Burki et al. Reference Burki, Roger, Brown and Simpson2020) and the endosymbiotically derived green lineage of dinoflagellates among alveolates (Margulis et al. Reference Margulis, Corliss, Melkonian and Chapman1989; Keeling, Reference Keeling2004; Raven et al. Reference Raven, Evert and Eichhorn2005; Falkowski & Raven, Reference Falkowski and Raven2007; Fehling et al. Reference Fehling, Stoecker, Baldauf, Falkowski and Knoll2007; O’Kelly, Reference O’Kelly, Falkowski and Knoll2007; Graham et al. Reference Graham, Graham and Wilcox2009; Leliaert et al. Reference Leliaert, Smith, Moreau, Herron, Verbruggen, Delwiche and De Clerck2012). In the Chloroplastida that have primary plastid originated directly from cyanobacterium, this common origin implies a strong morphological/cell biological synapomorphy (Burki et al. Reference Burki, Roger, Brown and Simpson2020). Alongside biochemical synthesis and certain enzymes acquired from early photosynthesizing ancestors, the genetic toolkits for reproduction and zygotic cyst formation were conceivably inherited and shared within the ‘green’ lineages of algae (including photosynthesizing dinoflagellates).
5.c. Cell division: palintomy
Palintomic cell division, the process during which a parental cell or zygote undergoes a rapid sequence of repeated divisions that result in decreased size of cells (Margulis et al. Reference Margulis, Corliss, Melkonian and Chapman1989, p. 778), has been observed in all microfossils studied here and some previously interpreted as animal embryos or holozoans (Xiao & Knoll, Reference Xiao and Knoll2000; Huldtgren et al. Reference Huldtgren, Cunningham, Yin, Stampanoni, Marone, Donoghue and Bengtson2011; but see Butterfield, Reference Butterfield2011; Donoghue et al. Reference Donoghue, Cunningham, Dong, Bengtson and Wanninger2015; Cunningham et al. Reference Cunningham, Vargas, Yin, Bengtson and Donoghue2017; Section 6.c). Palintomy in reproductive stage is common to unicellular green algae, protistan holozoans and metazoans but only at the early developmental stages up to 16-cell metazoan embryos (Margulis et al. Reference Margulis, Corliss, Melkonian and Chapman1989, pp. 610, 632; Mathews, Reference Mathews1986, pp. 24–5, 30–1; Gilbert & Raunio, Reference Gilbert and Raunio1997; Jurd, Reference Jurd2004; Lee, Reference Lee2008, pp. 192, 214, 217; Nielsen, Reference Nielsen2012; Leadbeater, Reference Leadbeater2015, p. 61). Looking at the pattern of cell divisions at the early stages alone, the microfossils cannot be discriminated between these clades. At the later ontogenetic stages with multiple cells that are identical and randomly clustered, microfossils may be considered among protistan holozoans and algae (Butterfield, Reference Butterfield2011; Huldtgren et al. Reference Huldtgren, Cunningham, Yin, Stampanoni, Marone, Donoghue and Bengtson2011). The decisive features in favour of either of these two are the cyst morphology (see Section 5.d) and, if possible to detect, the biochemistry or cyst wall properties.
The palintomy that is observed in Appendisphaera grandis, Mengeosphaera bellula and Tianzhushania spinosa is evident in cysts of the same size that contain one or four to multiple, and in Tianzhushania thousands of, identical cells (Figs 2a–c and 3a, b, e, f). Palintomic division is observed during sporogenesis in algae as well as in protistan holozoans and metazoans (Margulis et al. Reference Margulis, Corliss, Melkonian and Chapman1989; Van den Hoek et al. Reference Van den Hoek, Mann and Jahns1995; Jurd, Reference Jurd2004; Butterfield, Reference Butterfield2011; Huldtgren et al. Reference Huldtgren, Cunningham, Yin, Stampanoni, Marone, Donoghue and Bengtson2011). However, in algae and protistan holozoans, this process leads to formation of morphologically identical offspring cells (which may be very numerous; Margulis et al. Reference Margulis, Corliss, Melkonian and Chapman1989; Van den Hoek et al. Reference Van den Hoek, Mann and Jahns1995; Lee et al. Reference Lee, Leedale and Bradbury2000; Graham et al. Reference Graham, Graham and Wilcox2009; Leadbeater, Reference Leadbeater2015). In metazoans, palintomic division occurs only at the morula and early blastula stages, and thereafter the cells are programmed to follow routes to specification (Kessel & Shih, Reference Kessel and Shih1974; Gilbert, Reference Gilbert2010; Nielsen, Reference Nielsen2012; Shilo, Reference Shilo2014). In progressing cell divisions, the cells are differentiated in shape and size, oriented into poles, asymmetrically segregated and aligned and grow into tissues. In animal embryos, cell differentiation, their orientation and polarization begin from the stage of 16 cells and after the third round of cell divisions (Anderson, Reference Anderson1973; Kessel & Shih, Reference Kessel and Shih1974; Mathews, Reference Mathews1986; Gilbert & Raunio, Reference Gilbert and Raunio1997; Jurd, Reference Jurd2004; Gilbert, Reference Gilbert2010; Nielsen, Reference Nielsen2012; Shilo, Reference Shilo2014; Gross et al. Reference Gross, Treffkorn, Mayer and Wanninger2015). Even in tardigrades, which may not have obvious blastocoel and in which the sterroblastula is a ball of cells, the cells are differentiated in shape (Gross et al. Reference Gross, Treffkorn, Mayer and Wanninger2015; Levin et al. Reference Levin, Anavy, Alison, Cole, Winter, Mostov, Khair, Senderovich, Kovalev, Silver, Feder, Fernandez-Valverde, Nakanishi, Simmons, Simakov, Larsson, Liu, Jerafi-Vider, Yaniv, Ryan, Martindale, Rink, Arendt, Degnan, Degnan, Hashimshony and Yanai2016). The animal fertilization membrane or egg cyst is discarded and the embryo grows from a blastocyst to a gastrula stage and then into a larva (Mathews, Reference Mathews1986; Gilbert & Raunio, Reference Gilbert and Raunio1997; Jurd, Reference Jurd2004; Gilbert, Reference Gilbert2010). This developmental pattern is the current embryology dogma without exception in extant animals of any phylogenetic position, from sponges and cnidarians to higher phyla (Fig. 5 further below). None of the microfossils studied here or those previously inferred to be animal embryos (Tianzhushania and its putative developmental stages Megasphaera, Parapandorina and Megaclonophycus; Xiao & Knoll, Reference Xiao and Knoll2000; C Yin et al. Reference Yin, Bengtson and Yue2004; L Yin et al. Reference Yin, Zhu, Knoll, Yuan, Zhang and Hu2007, Reference Yin, Zhou and Yuan2008; Chen et al. Reference Chen, Xiao, Pang, Zhou and Yuan2014) have ever shown the presence of a blastocoel or gastrocoel or cell differentiation, which are animal embryonic characters, even in the thousands of cells stages (Hagadorn et al. Reference Hagadorn, Xiao, Donoghue, Bengtson, Gostling, Pawlowska, Raff, Raff, Turner, Yin, Zhou, Yuan, McFeely, Stampanoni and Nealson2006; Huldtgren et al. Reference Huldtgren, Cunningham, Yin, Stampanoni, Marone, Donoghue and Bengtson2011; Chen et al. Reference Chen, Xiao, Pang, Zhou and Yuan2014; Donoghue et al. Reference Donoghue, Cunningham, Dong, Bengtson and Wanninger2015; Cunningham et al. Reference Cunningham, Vargas, Yin, Bengtson and Donoghue2017). Cell differentiation claimed to occur in Megaclonophycus-like microfossils (Chen et al. Reference Chen, Xiao, Pang, Zhou and Yuan2014) is not substantiated and this record is alternatively interpreted (Tang, Reference Tang2016; see Section 6.c). New specimens of A. grandis, M. bellula and T. spinosa (Figs 2 and 3) preserved in the late developmental stages provide the decisive evidence to dismiss the animal embryo interpretation for Tianzhushania (Xiao & Knoll, Reference Xiao and Knoll2000; Yin et al. Reference Yin, Bengtson and Yue2004) and Appendisphaera (Yin et al. Reference Yin, Zhu, Knoll, Yuan, Zhang and Hu2007). Mengeosphaera is not different in this respect.
Palintomic cell division was inferred in peanut-shaped microfossils that were attributed to the protistan holozoan Tianzhushania life cycle (Huldtgren et al. Reference Huldtgren, Cunningham, Yin, Stampanoni, Marone, Donoghue and Bengtson2011; Donoghue et al. Reference Donoghue, Cunningham, Dong, Bengtson and Wanninger2015; Cunningham et al. Reference Cunningham, Vargas, Yin, Bengtson and Donoghue2017) and resulted in forming thousands of tightly arranged cells. However, these cells are not identical and are aligned into layers in the peripheral portion of the microfossil. This appears not to be strictly palintomic division, or the layers were formed after the palintomy ceased. In any case, the cell differentiation and layering in peanut-shaped microfossil is very different from that of Tianzhushania spinosa, and does not belong to the same life cycle (see section 6.c).
5.d. Cyst morphology
Cyst morphology, alongside pattern of cell division and cyst wall biochemistry, is a characteristic phenotypic feature. Morphologically complex cysts as observed in the studied microfossils are well-known among green microalgae (Bold & Wynne, Reference Bold and Wynne1985, pp. 93, 143, 167; Van den Hoek et al. Reference Van den Hoek, Mann and Jahns1995, pp. 352, 471; Hagen et al. Reference Hagen, Siegmund and Braune2002; Raven et al. Reference Raven, Evert and Eichhorn2005, pp. 296, 327, 337; Damiani et al. Reference Damiani, Leonardi, Pieroni and Cáceres2006; Lee, Reference Lee2008, pp. 152, 192–3; Graham et al. Reference Graham, Graham and Wilcox2009, pp. 434–5; Van Westen, Reference Van Westen2015; Figs 4 and 5) but are not produced by protistan holozoans (Leadbeater, Reference Leadbeater2015, pp. 47, 49, 61; Torruella et al. Reference Torruella, de Mendoza, Grau-Bové, Antó, Chaplin, del Camplo, Eme, Pérez-Cordón, Whipps, Nichols, Paley, Roger, Sitjà-Bobadilla, Donachie and Ruiz-Trillo2015; Adl et al. Reference Adl, Bass, Lane, Lukes, Schoch, Smirnov, Agatha, Berney, Brown, Burki, Cárdenas, Cepicka, Chistyakova, del Campo, Dunthorn, Edvardsen, Eglit, Guillou, Hampl, Heiss, Hoppenrath, James, Karnkowska, Karpov, Kim, Kolisko, Kudryavtsev, Lahr, Lara, Le Gall, Lynn, Mann, Massana, Mitchell, Morrow, Soo Park, Pawlowski, Powell, Richter, Rueckert, Shadwick, Shimano, Spiegel, Torruella, Youssef, Zlatogursky and Zhang2019) or the lower animal phyla known today and inferred to exist in the Ediacaran (sponge, cnidarians, placozoans, lophotrochozoans; Sperling & Vinther, Reference Sperling and Vinther2010; Zhuravlev et al. Reference Zhuravlev, Liñán, Gámez Vintaned, Debrenne and Fedorov2012; Wood et al. Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019; Fig. 5). The higher animal phyla such as tardigrades and arthropods that may form spinose cysts (Sanoamuang et al. Reference Sanoamuang, Saengphan and Murugan2002; Rabet, Reference Rabet2010; Scholtz & Wolff, Reference Scholtz, Wolff, Minelli, Boxhall and Fusco2013) had not yet evolved at the time, if relying on the fossil record (Budd & Jensen, Reference Budd and Jensen2000; Kouchinsky et al. Reference Kouchinsky, Bengtson, Runnegar, Skovsted, Steiner and Vendrasco2012) and in agreement with some molecular clock estimates (Erwin et al. Reference Erwin, Laflamme, Tweedt, Sperling, Pisani and Peterson2011). The earliest tardigrade fossils are middle Cambrian (Müller et al. Reference Müller, Walossek and Zakharov1995; Gross et al. Reference Gross, Treffkorn, Mayer and Wanninger2015) and arthropods are known from the early Cambrian (Fortunian Stage trace fossils, and Stage 3 body fossils; Erwin & Valentine, Reference Erwin and Valentine2013), thus not supporting comparisons of their cysts’ morphology with Ediacaran microfossils. Based on the new specimens and evaluating their features at the late reproductive stages, and as Tianzhushania spinosa has never before been observed at such multicellular stage, we further explore the possible affinities of cyst-like vesicles bearing processes and membranes.
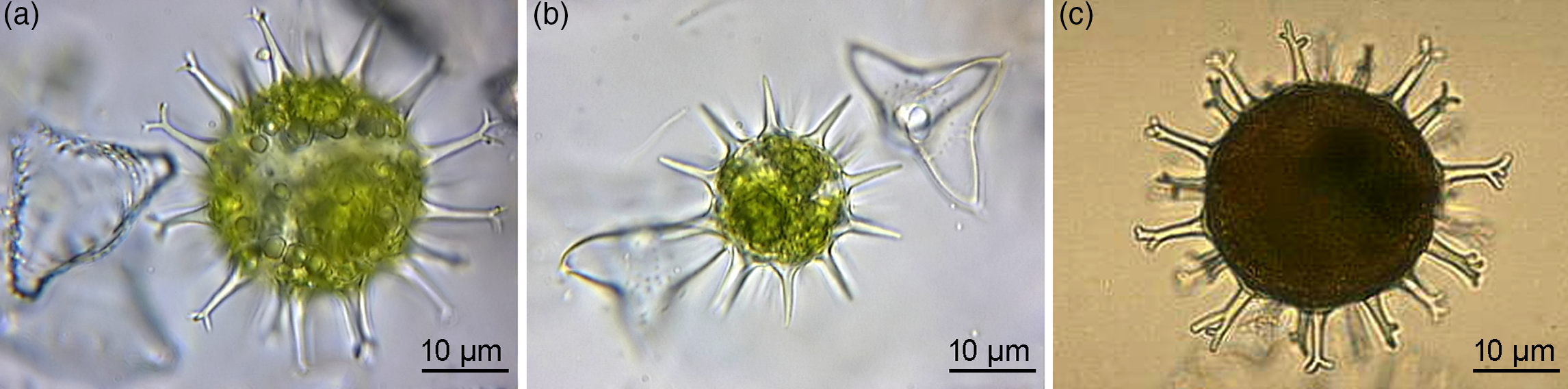
Fig. 4. Extant green algal (desmidiacean) zygotic cysts that are morphological counterparts to the studied microfossils. (a) Staurastrum borgeanum. (b) Staurodesmus dejectus. (c) Micrasterias papillifera. (a, b) Specimens from ponds in the Netherlands. (c) Algaebase, Galway, National University of Ireland, Online Collection (Guiry & Guiry, Reference Guiry and Guiry2019), http://algaebase.org. All are transmitted-light micrographs.
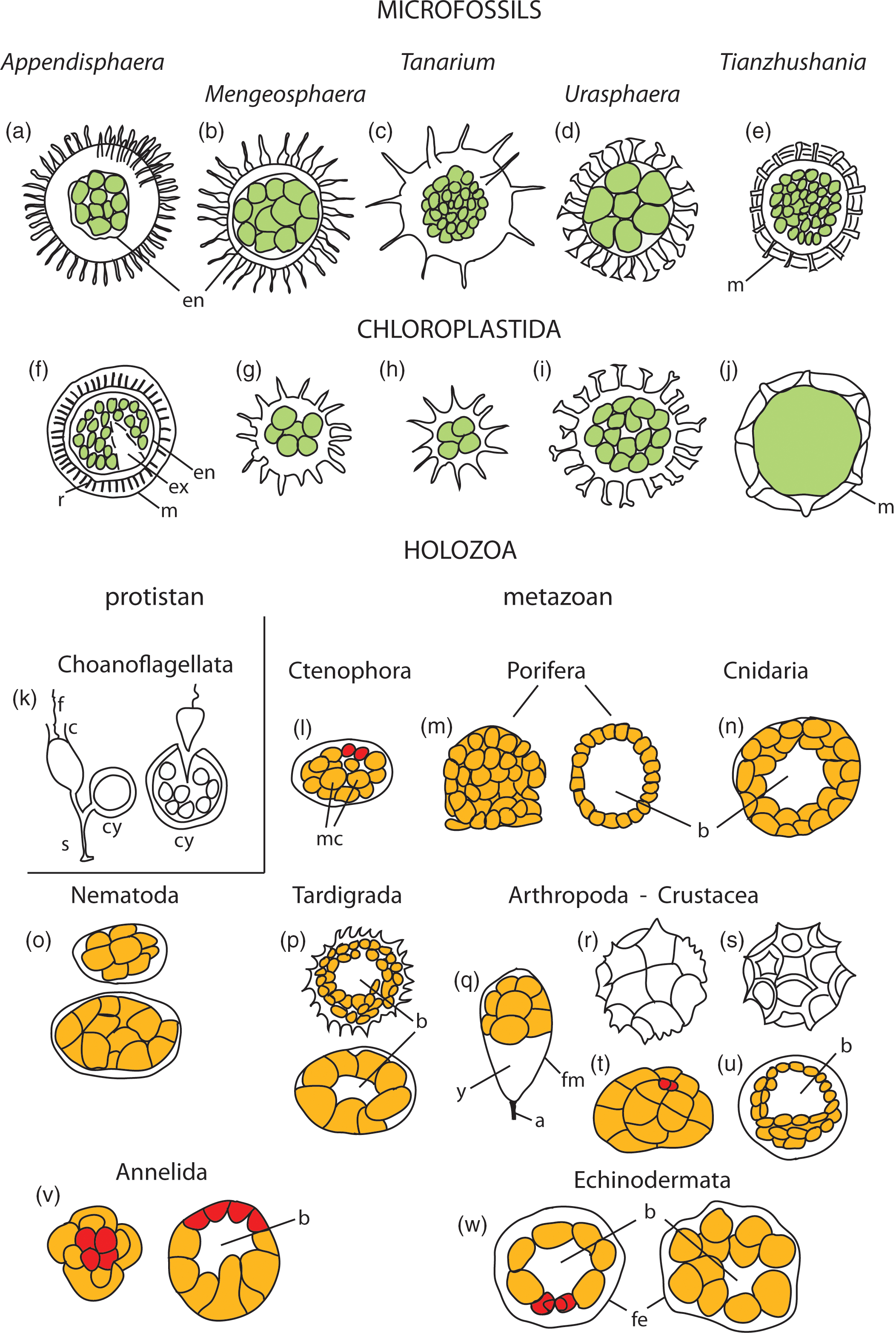
Fig. 5. Schematic comparative morphology of studied microfossils, reproductive cysts with offspring cells in Chloroplastida (green algae), and embryology of Holozoa, including eggs, developing embryos and diapause cysts. (a–e) Microfossils with processes- and external membranes-bearing (m) cyst-like vesicles containing endocyst (en) inside vesicle cavity and internal spheroidal cells of equal sizes and tightly clustered, numbering from four (Fig. 2a) to numerous to hundreds (T. spinosa) seen in vesicle sections. (f–j) Examples of reproductive cysts in the group Chloroplastida, showing morphologic pattern of overall shape and characteristic processes, external membranes (m), rod-like elements supporting membrane (r), excystment structure (ex) and endocysts (en), and containing palintomically dividing offspring cells (in green). (k–w) Embryos, diapause cysts and eggs of representative organisms from the Supergroup Holozoa, including protistan (unicellular) and metazoan (multicellular) holozoans. (k) Codosiga botrytis, stalked (s) cell with flagellum (f) and collar (c) and cyst (cy) that contains dividing cells and releases many small flagellated cells (after Leadbeater, Reference Leadbeater2015). (l–w) Metazoan holozoans; micromeres (mm) marked in red colour, macromeres (mc) in orange colour, blastcoel (b). Details in the Supplementary Material available online at https://doi.org/10.1017/S0016756820001405.
Among the body plan and described morphological features, the studied microfossil taxa show excystment structures (pylome in Appendisphaera and median split in others), which are characteristic of reproductive cysts in extant algae (Evitt, Reference Evitt1985; Dale, Reference Dale2001; Head et al. Reference Head, Lewis and De Vernal2006; Moczydłowska, Reference Agić, Moczydłowska and Canfield2016). These structures differ from structures in some heterotrophic protist cysts, such as in amoebae and ciliates, which are morphologically predetermined to be openings with collars, necks or rims, or open by lysis of the wall (Tappan, Reference Tappan and Lipps1993; Porter, Reference Porter, Xiao and Kaufman2006; Bosak et al. Reference Bosak, Macdonald, Lahr and Matys2011; Morais et al. Reference Morais, Fairchild, Lahr, Rudnitzki, Schopf, Garcia, Kudryavtsev and Romero2017), in addition to dissimilar shape of cyst. Diapause egg cysts in extant animals are opened by enzymatic autolysis of the cyst wall and are discarded without any morphologically defined opening structure (Gilbert & Raunio, Reference Gilbert and Raunio1997; Jurd, Reference Jurd2004). For our interpretation, however, we analysed not one, the excystment, but a combined set of features to recognize the possible phylogenetic relationships between microfossils and extant biota. The described individual features (shape of processes, their sizes and distribution) in various combinations in ornamented vesicles with occasionally preserved openings and with resistant walls, as well as newly observed reproductive cells and their spatial arrangements, as observed in Appendisphaera, Urasphaera, Mengeosphaera and Tanarium, are representative for their affinities and are found in extant microalgae. The set of morphological features expressed in studied taxa are consistent with the overall morphology of reproductive cysts (Bold & Wynne, Reference Bold and Wynne1985, pp. 93, 143, 167; Van den Hoek et al. Reference Van den Hoek, Mann and Jahns1995, pp. 352, 364, 471; Hagen et al. Reference Hagen, Siegmund and Braune2002; Raven et al. Reference Raven, Evert and Eichhorn2005, pp. 331, 337; Damiani et al. Reference Damiani, Leonardi, Pieroni and Cáceres2006; Graham et al. Reference Graham, Graham and Wilcox2009, pp. 414, 434–5, 464; Van Westen, Reference Van Westen2015; Guiry & Guiry, Reference Guiry and Guiry2019; see summary by Moczydłowska, Reference Agić, Moczydłowska and Canfield2016; Figs 4 and 5).
A morphological element that is peculiar to Tianzhushania – the external multilayered or multilamellar membrane surrounding the cyst (Fig. 3e) – has not been considered as a possible indicative feature, neither in the animal nor protistan holozoan interpretations. Such morphology is unknown in egg-cases, diapause cysts or any reproductive stages in animals or protistan holozoans (Gilbert & Raunio, Reference Gilbert and Raunio1997; Lee et al. Reference Lee, Leedale and Bradbury2000; Jurd, Reference Jurd2004; Gilbert, Reference Gilbert2010; Leadbeater, Reference Leadbeater2015; Adl et al. Reference Adl, Bass, Lane, Lukes, Schoch, Smirnov, Agatha, Berney, Brown, Burki, Cárdenas, Cepicka, Chistyakova, del Campo, Dunthorn, Edvardsen, Eglit, Guillou, Hampl, Heiss, Hoppenrath, James, Karnkowska, Karpov, Kim, Kolisko, Kudryavtsev, Lahr, Lara, Le Gall, Lynn, Mann, Massana, Mitchell, Morrow, Soo Park, Pawlowski, Powell, Richter, Rueckert, Shadwick, Shimano, Spiegel, Torruella, Youssef, Zlatogursky and Zhang2019). In extant algae, the external membrane is a common feature of the cyst in the initial process of its formation, during which the wall is secreted inside the membrane as the primary and secondary wall and thus may be multilayered and have a complex ultrastructure (Schlösser, Reference Schlösser, Irving and John1984; Bold & Wynne, Reference Bold and Wynne1985; Kokinos & Anderson, Reference Kokinos and Anderson1995; Dale, Reference Dale2001; Hagen et al. Reference Hagen, Siegmund and Braune2002; Damiani et al. Reference Damiani, Leonardi, Pieroni and Cáceres2006). The external membrane is present in several resistant organic-walled microfossils, which have been identified as possible algal cysts and of much older ages, at the time when animals had certainly not yet appeared (i.e. in the Mesoproterozoic; see dos Reis et al. Reference Dos Reis, Thawornwattana, Angelis, Telford, Donoghue and Yang2015). A few heterotrophic protists (amoebozoans and cercozoans) known in the Tonian (Porter, Reference Porter, Xiao and Kaufman2006) do not possess any membranes. Mesoproterozoic Shuiyousphaeridium and Gigantosphaeridium (Agić et al. Reference Agić, Moczydłowska and Yin2015, Reference Agić, Moczydłowska and Yin2017) and the Tonian Trachyhystrichosphaera and Cymatiosphaeroides (Vidal & Ford, Reference Vidal and Ford1985; Butterfield et al. Reference Butterfield, Knoll and Sweet1994) are examples of microfossils with external membranes. Cymatiosphaeroides has a single to multilaminated membranous envelope supported by processes and vesicle diameter ranging from 30 to 350 μm (Butterfield et al. Reference Butterfield, Knoll and Sweet1994, fig. 15c), and a body plan like that of Tianzhushania. These features are phenotypically consistent with some green algal cysts (Fig. 5). Some Mesoproterozoic taxa have been interpreted as stem-group Chloroplastida (Moczydłowska et al. Reference Moczydłowska, Landing, Zang and Palacios2011; Agić et al. Reference Agić, Moczydłowska and Yin2015), and Cymatiosphaeroides as chlorophycean alga (Moczydłowska, Reference Agić, Moczydłowska and Canfield2016).
5.e. Reproductive cycle
In the studied species, the cysts with one zygotic to four and multiple dividing cells show a morphological pattern that is characteristic of green algal cysts containing dividing offspring cells (spores) (Schlösser, Reference Schlösser, Irving and John1984; Bold & Wynne, Reference Bold and Wynne1985, pp. 129, 133, 141; Van den Hoek et al. Reference Van den Hoek, Mann and Jahns1995, pp. 352, 364, 366, 471; Yamamoto et al. Reference Yamamoto, Nozaki and Miyzawa2003; Raven et al. Reference Raven, Evert and Eichhorn2005, pp. 331, 327, 337; Lee, Reference Lee2008, p. 217; Graham et al. Reference Graham, Graham and Wilcox2009, pp. 414, 434, 464; Van Westen & Coesel, Reference Van Westen and Coesel2014). The offspring cells (aplanospores, autospores, spores, swarmers) may be numerous (32–64 to hundreds; above citations) within algal cysts and are not limited to any strict number. The lack of a cavity in the multiple-celled clusters is dissimilar to animal embryos, which would have a blasto- or gastrocoel in multiple-celled developing stages as well as differentiated cells arranged into layers and poles (Kessel & Shih, Reference Kessel and Shih1974; Mathews, Reference Mathews1986; Jurd, Reference Jurd2004; Gilbert, Reference Gilbert2010; Nielsen, Reference Nielsen2012; Fig. 5). The absence of these diagnostic animal embryonic characters indicates that the studied microfossils are not of animal origin. In particular, this interpretation opposes the placement of Appendisphaera tenuis among animal diapause egg cysts (Yin et al. Reference Yin, Zhu, Knoll, Yuan, Zhang and Hu2007).
The new record of multiple identical cells of various numbers within specimens of the same species documents the ontogenetic stages as is seen in unicellular green algal maturing cysts (Bold & Wynne, Reference Bold and Wynne1985; Van den Hoek et al. Reference Van den Hoek, Mann and Jahns1995; Hagen et al. Reference Hagen, Siegmund and Braune2002; Raven et al. Reference Raven, Evert and Eichhorn2005; Damiani et al. Reference Damiani, Leonardi, Pieroni and Cáceres2006; Lee, Reference Lee2008). The extant unicellular green algae show, in general, a similarly simple mode of reproduction forming multiple equal offspring cells tightly clustered inside the cyst, and this pattern might have been followed from the ‘generalist ancestor’ because immediate living relatives are hitherto unknown (Torruella et al. Reference Torruella, de Mendoza, Grau-Bové, Antó, Chaplin, del Camplo, Eme, Pérez-Cordón, Whipps, Nichols, Paley, Roger, Sitjà-Bobadilla, Donachie and Ruiz-Trillo2015).
In the cyst of Tianzhushania spinosa, the offspring cells were formed in the membranous, smooth-walled endocyst within its cavity. The offspring cells were likely released through the rupture in the cyst wall and endocyst and when freed they began to grow to vegetative cells. This life cycle included vegetative cells which are unrepresented as fossils, and only one kind of reproductive stage of which is the cyst represented by T. spinosa. This is because thousands of offspring cells indicating the late developmental stage were produced directly in the T. spinosa cyst. This evidence does not support the previous interpretations on the presence of several intervening developmental stages with different morphology in the life cycle of Tianzhushania (see Section 6.c). The T. spinosa cyst was likely zygotic and formed around the two fused (mating) vegetative cells of opposite orientation, e.g. + – strains, as it is known in algae (Van den Hoek et al. Reference Van den Hoek, Mann and Jahns1995, p. 352; Raven et al. Reference Raven, Evert and Eichhorn2005, p. 331; Lee, Reference Lee2008, p. 192; Graham et al. Reference Graham, Graham and Wilcox2009, p. 375). The diploidal zygote first divided meiotically to return to haploidal cells characteristic of algae, and then mitotically and palintomically in a series of divisions producing offspring cells (spores). After the cyst matured and contained the critical mass of cells, which reached the minimum viable size, they were released and the life cycle was closed. Based on the present record, new observations and the evaluation of previous interpretations of Tianzhushania as metazoan or protistan holozoan (see Section 6.c), we propose algal affinity for this taxon.
5.f. Comparisons to modern algae
The cyst morphology and reproductive cycle in various lineages of extant algae, which are well-recognized and accepted knowledge substantiated by new case studies (Van den Hoek et al. Reference Van den Hoek, Mann and Jahns1995; Hagen et al. Reference Hagen, Siegmund and Braune2002; Yamamoto et al. Reference Yamamoto, Nozaki and Miyzawa2003; Raven et al. Reference Raven, Evert and Eichhorn2005; Damiani et al. Reference Damiani, Leonardi, Pieroni and Cáceres2006; Lee, Reference Lee2008; Graham et al. Reference Graham, Graham and Wilcox2009; Van Westen & Coesel, Reference Van Westen and Coesel2014; Van Westen Reference Van Westen2015; see the summary in Moczydłowska, Reference Agić, Moczydłowska and Canfield2016; Fig. 5), provide striking phenotypic analogues to the microfossils studied. These analogues are found in the group Chloroplastida, in its basal division Chlorophyta and derived Streptophyta (according to classification by Adl et al. Reference Adl, Bass, Lane, Lukes, Schoch, Smirnov, Agatha, Berney, Brown, Burki, Cárdenas, Cepicka, Chistyakova, del Campo, Dunthorn, Edvardsen, Eglit, Guillou, Hampl, Heiss, Hoppenrath, James, Karnkowska, Karpov, Kim, Kolisko, Kudryavtsev, Lahr, Lara, Le Gall, Lynn, Mann, Massana, Mitchell, Morrow, Soo Park, Pawlowski, Powell, Richter, Rueckert, Shadwick, Shimano, Spiegel, Torruella, Youssef, Zlatogursky and Zhang2019; Burki et al. Reference Burki, Roger, Brown and Simpson2020). Modern morphological counterparts are observed in cysts of numerous marine species of basal chlorophytes, such as Chlamydomonas and Golenkinia (Bold & Wynne, Reference Bold and Wynne1985; Van den Hoek et al. Reference Van den Hoek, Mann and Jahns1995; Raven et al. Reference Raven, Evert and Eichhorn2005; Falkowski & Raven, Reference Falkowski and Raven2007; Fehling et al. Reference Fehling, Stoecker, Baldauf, Falkowski and Knoll2007; O’Kelly, Reference O’Kelly, Falkowski and Knoll2007; Guiry, Reference Guiry2013) and in derived lineages of some freshwater streptophytes, such as Closterium, Cosmarium, Staurastrum, Staurodesmus and Micrasterias (Bold & Wynne, Reference Bold and Wynne1985; Van den Hoek et al. Reference Van den Hoek, Mann and Jahns1995; Lee, Reference Lee2008; Graham et al. Reference Graham, Graham and Wilcox2009; Van Westen & Coesel, Reference Van Westen and Coesel2014; Van Westen, Reference Van Westen2015; Figs 4 and 5). For example, the zygotic cyst of Chlamydomonas, which is thick-walled, resistant and ornamented by spines, contains a single large cell, the zygote, which after multiple divisions produces numerous, small spheroidal offspring cells (Schlösser, Reference Schlösser, Irving and John1984; Bold & Wynne, Reference Bold and Wynne1985; Van den Hoek et al. Reference Van den Hoek, Mann and Jahns1995; Raven et al. Reference Raven, Evert and Eichhorn2005; Lee, Reference Lee2008). If fossilized, Chlamydomonas would show a morphological pattern similar to some of the studied microfossils.
Further morphological analogues, including three genera of extant desmidiacean algae in the streptophytes, exemplify the zygotic cyst morphology of acanthomorphic vesicles with conical simple or divided process tips and a wall composed of refractory biopolymers (Figs 4a–c and 5). The cyst encloses spheroidal dividing cells (spores), initially two as found in Staurodesmus (Fig. 4b), to multiple, as illustrated in Staurastrum and Micrasterias (Fig. 4a, c). At the early developmental stage, the zygote forms a single internal body, and the cyst still contains chlorophyll (Fig. 4a, b) and is metabolically active, not only in the process of zygotic subdivision. The mature cyst is devoid of chlorophyll (Fig. 4c). Despite being freshwater representatives, these algae cannot be excluded from comparisons since algae either originated from marine ancestors (Falkowski & Raven, Reference Falkowski and Raven2007; Fehling et al. Reference Fehling, Stoecker, Baldauf, Falkowski and Knoll2007; Hackett et al. Reference Hackett, Yoon, Butterfield, Sanderson, Bhattacharya, Falkowski and Knoll2007; Knoll et al. Reference Knoll, Summons, Waldbauer, Zumberge, Falkowski and Knoll2007) or in low-salinity habitats (Sánchez-Baracaldo et al. Reference Sánchez-Baracaldo, Raven, Pisani and Knoll2017). However, the latter habitat is not reconciled with the fossil record and is exemplified by the habitats of modern taxa. These habitats are ephemeral, contrary to the expected robustness of the system to sustain the evolving biota. It is also well-known that even the same genus, such as prasinophycean extant algae and fossil Cymatiosphaera, may occupy marine, brackish and freshwater environments preserving the same morphology (Tappan, Reference Tappan1980; Dotzel et al. Reference Dotzel, Taylor and Krings2007).
The combined features of resistant organic-walled vesicles with surface ornamentation in the form of processes and/or membranes, an excystment opening, an internal membranous endocyst containing a single to multiple cells, and the evidence of multiple palintomically dividing cells with wall furrows that are preserved inside the vesicle cavity are consistent with inferring that the studied microfossils represent zygotic reproductive cysts of green algae. The earlier interpretation of Appendisphaera and Tanarium as representing algal zygotic cysts (Moczydłowska, Reference Moczydłowska2005, Reference Agić, Moczydłowska and Canfield2016; Moczydłowska et al. Reference Moczydłowska, Landing, Zang and Palacios2011) or conventionally existing among phytoplankton (Grey, Reference Grey2005) and made prior to observing internal cells is now reinforced by evidence of reproductive stages with cell division diagnostic of algae.
5.g. Comparisons to microfossils of other ages
Ediacaran cysts are, in all the features analysed here, similar to many microfossil species of various ages from the Mesoproterozoic to Palaeozoic, which were inferred to be algal in affinity based on comparative morphology with extant taxa (Tappan, Reference Tappan1980; Zang & Walter, Reference Zang and Walter1989; Moczydłowska, Reference Moczydłowska1991, Reference Moczydłowska2005, Reference Moczydłowska2010, Reference Agić, Moczydłowska and Canfield2016; Moczydłowska et al. Reference Moczydłowska, Vidal and Rudavskaya1993, Reference Moczydłowska, Landing, Zang and Palacios2011; Colbath & Grenfell, Reference Colbath and Grenfell1995; Arouri et al. Reference Arouri, Greenwood and Walter2000; Grey, Reference Grey2005; Willman & Moczydłowska, Reference Willman and Moczydłowska2007; Lamb et al. Reference Lamb, Awramik, Chapman and Zhu2009; Moczydłowska & Willman, Reference Moczydłowska and Willman2009; Agić et al. Reference Agić, Moczydłowska and Yin2015, Reference Agić, Moczydłowska and Canfield2016, Reference Agić, Moczydłowska and Yin2017; Miao et al. Reference Miao, Moczydłowska, Zhu and Zhu2019; Shang et al. Reference Shang, Liu, Moczydłowska and Yang2020). This similarity between fossil cysts and extant algal cysts is argued not to be coincidental or the result of convergent morphology in polyphyletic organisms, but the expression of phylogenetic relationships within some green algal lineages from their early ancestors to those living today. As mentioned above, the extant algae inherited and share their mode of reproduction with related and early diverging lineages, along with the biosynthesis of polymers that comprise resistant cyst walls and some enzymes metabolically vital for photosynthesis (Raven et al. Reference Raven, Evert and Eichhorn2005; David & Alm, Reference David and Alm2011). The sexual reproduction and biosynthesis of refractory polymers in the walls of protective cysts harbouring zygote and dividing cells acquired in the deep phylogenetic history by algae in marine environments was an evolutionary success due to certain directly and endosymbiotically inherited features (Margulis et al. Reference Margulis, Corliss, Melkonian and Chapman1989; Woese et al. Reference Woese, Kandler and Wheelis1990; Falkowski & Raven, Reference Falkowski and Raven2007; O’Kelly, Reference O’Kelly, Falkowski and Knoll2007; Torruella et al. Reference Torruella, de Mendoza, Grau-Bové, Antó, Chaplin, del Camplo, Eme, Pérez-Cordón, Whipps, Nichols, Paley, Roger, Sitjà-Bobadilla, Donachie and Ruiz-Trillo2015; Burki et al. Reference Burki, Roger, Brown and Simpson2020), as were some biochemical processes and the ability to form zygotic cysts. These best-fitted characters in reproductive cysts might have been selected for and shared in photosynthetic algae that may be traced back by microfossils to c. 1.7 Ga (Moczydłowska et al. Reference Moczydłowska, Landing, Zang and Palacios2011; Agić et al. Reference Agić, Moczydłowska and Yin2015, Reference Agić, Moczydłowska and Yin2017; Moczydłowska, Reference Agić, Moczydłowska and Canfield2016; Miao et al. Reference Miao, Moczydłowska, Zhu and Zhu2019). The evolutionary history of green algae, the chlorophytes, if accepting the affinity of such microfossils, may be inferred from the fossil record in marine environments beginning prior to c. 1.7 Ga and that of phylogenetically more distant streptophytes adapted to freshwater environments prior to c. 1.0 Ga (Raven et al. Reference Raven, Evert and Eichhorn2005; Knauth & Kennedy, Reference Knauth and Kennedy2009). Microfossil algal affinities (Moczydłowska, Reference Agić, Moczydłowska and Canfield2016; Loron & Moczydłowska, Reference Loron and Moczydłowska2018) are disputed (Javaux & Knoll, Reference Javaux and Knoll2017; Del Cortana et al. Reference Del Cortana, Jackson, Bucchini, Van Bel, D’hondt, Škaloud, Delwiche, Knoll, Raven, Verbruggen, Vandepoele, De Cleck and Leliaert2020), so microfossil taxa may be selectively used to calibrate molecular clock estimates, resulting in younger estimates for the divergence of Chlorophyta, e.g. c. 1 Ga (Del Cortana et al. Reference Del Cortana, Jackson, Bucchini, Van Bel, D’hondt, Škaloud, Delwiche, Knoll, Raven, Verbruggen, Vandepoele, De Cleck and Leliaert2020). This estimate has not considered more recent records (Agić et al. Reference Agić, Moczydłowska and Yin2015, Reference Agić, Moczydłowska and Yin2017; Miao et al. Reference Miao, Moczydłowska, Zhu and Zhu2019) for fossil calibration, or dealt only with the multicellular algae (Tang et al. Reference Tang, Pang, Yuan and Xiao2020). The wide timespans for divergence of Archaeoplastida at c. 1.6–1.1 Ga and the symbiotic origin of the plastid at c. 1.7–1.1 Ga (Betts et al. Reference Betts, Puttick, Clark, Williams and Donoghue2018) may account for uncertainties in the methods used for estimates and are not precise (see also dos Reis et al. Reference Dos Reis, Thawornwattana, Angelis, Telford, Donoghue and Yang2015). Mis-calibration of the molecular clock estimates is affected by the difficulty in interpreting fossils with no extant exemplars and the reconstruction of ancestral characters (Leliaert et al. Reference Leliaert, Smith, Moreau, Herron, Verbruggen, Delwiche and De Clerck2012).
The c. 1.7 Ga record of green algae, if further confirmed, broadly coincides with that of red algae at c. 1.6 Ga, which however diverged earlier according to the phylogenomics of green plants (Corlett et al. Reference Corlett2019). Taking fossil record biases into account, both the red and green algae are still very ancient organisms. The red algae, the rhodophytes, represented by the putative florideophycean and bangiophycean classes, are recognized in the fossil record in the 1.0 Ga Hunting Formation, Canada (Butterfield et al. Reference Butterfield, Knoll and Sweet1990; Butterfield, Reference Butterfield2000; see Gibson et al. Reference Gibson, Shih, Cumming, Fischer, Crockford, Hodgskiss, Wörndle, Creaser, Rainbird, Skulski and Halverson2018 for age 1.0 Ga), and the 1.6 Ga Lower Vindhyan Supergroup in India and are classed among crown-group eukaryotes (Bengtson et al. Reference Bengtson, Sallsted, Belivanova and Whitehouse2017; but cf. Betts et al. Reference Betts, Puttick, Clark, Williams and Donoghue2018, who suggests they belong to total group Archaeoplastida instead, and concerning age see also Ray, Reference Ray2006). Multicellular red, brown and/or green algae with parenchymatous thalli are also well-represented in the Ediacaran Doushantuo Formation (Steiner, Reference Steiner1994; Xue et al. Reference Xue, Tang, Yu and Zhou1995; Xiao et al. Reference Xiao, Zhang and Knoll1998, Reference Xiao, Knoll, Zhang and Hua1999, Reference Xiao2004; Xiao, Reference Xiao2002; Yuan et al. Reference Yuan, Xiao, Yin, Knoll, Zhou and Mu2002, Reference Yuan, Chen, Xiao, Zhou and Hua2011; Yin et al. Reference Yin, Zhu, Tafforeau, Chen, Liu and Li2013).
6. Discussion
6.a. Cyst size and number of offspring cells
Many Ediacaran microfossil taxa have wide size ranges, including those discussed. Appendisphaera grandis has a range of 50–812 μm diameter, Tianzhushania spinosa 350–980 μm, whereas Tanarium paucispinosum is only 83–198 μm and Mengeosphaera bellula 50–90 μm. All, however, contain numerous internal cells. A wide size range may be considered for inferring the life cycle of biological species with cyst reproductive stage and possible limitations of its size.
Size matters in all organismal clades for metabolically viable cells and their functions, and green unicellular microalgae are good examples of this, yet exceptional size ranges are found within all extant taxonomic groups (Bonner, Reference Bonner2006). Conventionally conceived as the smallest organisms, bacteria may reach giant sizes, such as the sulphur bacterium Tiomargarita being 100–750 μm in diameter (Bailey et al. Reference Bailey, Joye, Kalanetra, Flood and Corsetti2007 a), while at the opposite extreme the marine chlorophycean alga Nanochlorum eucaryotum is only of 1.5 μm average diameter (Wilhelm et al. Reference Wilhelm, Eisenbeis, Wild and Zahn1982; Sogin, Reference Sogin and Bengtson1994). Extant green microalgae (Chloroplastida, Chlorophyta; Adl et al. Reference Adl, Bass, Lane, Lukes, Schoch, Smirnov, Agatha, Berney, Brown, Burki, Cárdenas, Cepicka, Chistyakova, del Campo, Dunthorn, Edvardsen, Eglit, Guillou, Hampl, Heiss, Hoppenrath, James, Karnkowska, Karpov, Kim, Kolisko, Kudryavtsev, Lahr, Lara, Le Gall, Lynn, Mann, Massana, Mitchell, Morrow, Soo Park, Pawlowski, Powell, Richter, Rueckert, Shadwick, Shimano, Spiegel, Torruella, Youssef, Zlatogursky and Zhang2019) are relatively small and their vegetative cell dimensions are 20–200 μm with exceptions up to 2–20 mm (Van den Hoek et al. Reference Van den Hoek, Mann and Jahns1995; Raven et al. Reference Raven, Evert and Eichhorn2005; Graham et al. Reference Graham, Graham and Wilcox2009; Van Westen & Coesel, Reference Van Westen and Coesel2014). Yet the reproductive cysts (phycomata) in phylogenetically basal prasinophyceans are 100–800 μm (Tappan, Reference Tappan1980; Van den Hoek et al. Reference Van den Hoek, Mann and Jahns1995) and reproductive colonies of the chlorophycean Volvox are over 300 μm in diameter (Graham et al. Reference Graham, Graham and Wilcox2009; Nedelcu & Michod, Reference Nedelcu, Michod, Flatt and Heyland2012). In the life cycle of extant chlorophyte microalgae, the reproductive stages may contain multiple and numerous (tens to hundreds) offspring cells in asexual and zygotic cysts, such as in Chlorococcum or Chlamydomonas (Van den Hoek et al. Reference Van den Hoek, Mann and Jahns1995; Lee, Reference Lee2008), or Derbesia (Graham et al. Reference Graham, Graham and Wilcox2009). In the reproductive colonies of Volvox, the number of offspring cells reaches up to 500 or several thousand spheroidal cells (Graham et al. Reference Graham, Graham and Wilcox2009; Butterfield, Reference Butterfield2011; Guiry & Guiry, Reference Guiry and Guiry2019). The observed dimensions of the studied microfossil species and the abundance of reproductive cells are approximately commensurate with those known in cysts of extant green microalgae and do not exclude these microfossils from being of algal affinity, which is supported by their morphology and reproductive pattern.
The microfossil dimensions are neither indicative of animal egg capsules and diapause cysts, as was argued for the animal embryo interpretation (Xiao, Reference Xiao2002), nor as being typical of non-metazoan holozoans (= protists) (Huldtgren et al. Reference Huldtgren, Cunningham, Yin, Stampanoni, Marone, Donoghue and Bengtson2011; Donoghue et al. Reference Donoghue, Cunningham, Dong, Bengtson and Wanninger2015). The sizes of animal egg capsules and embryos are not universally large and can be also tiny. For example, the molluscan bivalve mussel Mytilus has an embryo at the developmental stage of sterroblastula only 60–65 μm long, a larval trochophore 70–75 μm long and a later larva stage 100–150 μm long (Dyachuk & Odintsova, Reference Dyachuk and Odintsova2009). Microscopic tardigrades, which are widely accepted as panarthropods, usually do not exceed 500 μm or 1 mm in length and produce resistant egg capsules and cysts containing eggs that are only 50–75 μm, while the late gastrula containing c. 500 cells is c. 110 μm long (Gross et al. Reference Gross, Treffkorn, Mayer and Wanninger2015). Exceptional size ranges are not restricted to the developmental stages but are also known in animal bodies. In arthropods, arachnids may be microscopic and only 80 μm in mites (Rubin et al. Reference Rubin, Young, Wright, Whitaker and Ahn2016). In contrast, scorpions can reach 23 cm in length (Rubio, Reference Rubio2000). Among protists, the size ranges may be enormous, as in foraminiferans which range from 100 μm to 20 cm (Loeblich & Tappan, Reference Loeblich and Tappan1964; Wetmore, Reference Wetmore2019). Size alone is not a diagnostic feature of microfossils or for discriminations any taxonomic group.
We hypothesize that the dimensions of the studied microfossils exemplify an evolutionary peculiarity of the Ediacaran microorganisms. It is clearly observed that, in general, the Ediacaran microfossils (accepted to be cysts regardless of their affinities) are larger than those of many organic-walled morphological counterparts in the Palaeozoic and all informally called acritarchs (Zang & Walter, Reference Zang and Walter1992; Moczydłowska et al. Reference Moczydłowska, Vidal and Rudavskaya1993; Grey, Reference Grey2005; Moczydłowska, Reference Moczydłowska2005; Willman et al. Reference Willman, Moczydłowska and Grey2006; Sergeev et al. Reference Sergeev, Knoll and Vorobeva2011; Liu et al. Reference Liu, Xiao, Yin, Chen, Zhou and Li2014; Xiao et al. Reference Xiao, Zhou, Liu, Wang and Yuan2014; Liu & Moczydłowska, Reference Liu and Moczydłowska2019). This phenomenon of ‘giantism’ may be attributed to eco-phenotypes, responding to environmental conditions of warm, nutrient-rich, well-oxygenated (at least surface layers) marine sea waters in the aftermath of global glacial intervals (Grey, Reference Grey2005; Moczydłowska, Reference Moczydłowska2005, Reference Moczydłowska2008 a, Reference Agić, Moczydłowska and Canfield2016; Canfield et al. Reference Canfield, Poulton and Narbonne2007; Hoffman et al. Reference Hoffman, Abbot, Askenazy, Benn, Brocks, Cohen, Cox, Creveling, Donnadieu, Erwin, Fairchild, Ferreira, Goodman, Halverson, Jansen, Le Hir, Love, Macdonald, Maloof, Partin, Ramstein, Rose, Rose, Sadler, Tziperman, Voigt and Warren2017). This is also observed in macroscopic fossils preserved by impressions, including metazoans of this period (Liu et al. Reference Liu, Kenchington and Mitchell2015; Hoyal et al. Reference Hoyal Cuthill and Han2018). The large sizes, ranging from decimetres to over metre-long, are characteristic of many groups, notably rangeomorphs, erniettomorphs and dickinsoniomorphs, which are metazoans and attributed to clades represented today by ‘normal’ (present standard) or small-sized organisms (Sperling & Vinther, Reference Sperling and Vinther2010; Narbonne et al. Reference Narbonne, Xiao, Shields, Gradstein, Ogg, Schmitz and Ogg2012; Vickers-Rich et al. Reference Vickers-Rich, Ivantsov, Trusler, Narbonne, Hall, Wilson, Greentree, Fedonkin, Elliott, Hoffman and Schneider2013; Liu et al. Reference Liu, Kenchington and Mitchell2015; Hoyal et al. Reference Hoyal Cuthill and Han2018; Wood et al. Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019). Ediacaran placozoan Dickinsonia that is up to several decimetres or at the extreme 2 m long (Sperling & Vinther, Reference Sperling and Vinther2010; Wood et al. Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019; or within a broad group of Eumetazoa plus Placozoa by Hoekzema et al. Reference Hoekzema, Brasier, Dunn and Liu2017) is a giant compared to extant analogue Trichoplax, which is usually 1–2 mm long (Nielsen, Reference Nielsen2012). The rangeomorphs are epifaunal (Narbonne et al. Reference Narbonne, Laflamme, Trusler, Darlymple and Greentree2014) and cnidarian in affinity (Wood et al. Reference Wood, Liu, Bowyer, Wilby, Dunn, Kenchington, Hoyal Cuthill, Michell and Penny2019), while modern representatives are generally small (Nielsen, Reference Nielsen2012).
The Gulliver (unusually large in size) vs Lilliput (exceptionally small in size) biotas are related to environmental conditions and are known in most modern phyla (Bonner, Reference Bonner2006) and their ancient representatives. The environmental-phenotype function and phenotypic plasticity is well-recognized in invertebrates and expressed by morphological response in life history (Flatt & Heyland, Reference Flatt, Heyland, Flatt and Heyland2012; Miner, Reference Miner, Flatt and Heyland2012), and in vertebrates, in which trait changes are a function of environmental variation (Hau & Wingfield, Reference Hau, Wingfield, Flatt and Heyland2012). In microfossils interpreted to be algae, the vesicle may be minute as in the early Cambrian Reticella and 4–10 μm in diameter (Agić, Reference Agić2015), or large as in the Mesoproterozoic Giganthosphaeridium up to 595 μm in diameter (Agić et al. Reference Agić, Moczydłowska and Yin2015) and the Ediacaran Appendisphaera up to 812 μm in diameter (herein). Thus, the large sizes of vesicles of the Ediacaran microfossils may not be a surprise or may not preclude the possibility of representing microalgal cysts, even if extant representatives are on average smaller. The larger the cyst, the larger the number of offspring cells it may produce.
6.b. Other Ediacaran microfossils
Some other Ediacaran microfossils with cyst-like morphology, such as Alicesphaeridium sp. and Gyalosphaeridium sp. and a few unnamed taxa, were thought to be animal resting cysts (Cohen et al. Reference Cohen, Knoll and Kodner2009), although without demonstrating any characteristic features restricted to animal cysts or embryonic cells. This assumption was questioned (Moczydłowska et al. Reference Moczydłowska, Landing, Zang and Palacios2011) because it was based on artefacts of microtome cutting marks in transmission electron microscope (TEM) images that were interpreted as wall ultrastructure features seen in microfossil and modern animal eggs. These features contrasted with the vesicle wall ultrastructure previously documented in the type species Gyalosphaeridium pulchrum (Willman & Moczydłowska, Reference Willman and Moczydłowska2007; Moczydłowska & Willman, Reference Moczydłowska and Willman2009).
Specifically, the wall ultrastructure of the resting cyst of the extant arthropod Branchinella (brine shrimp) seen in TEM images was suggested to be three-layered and similar to the vesicle wall of Gyalosphaeridium sp., thus showing a common affinity (Cohen et al. Reference Cohen, Knoll and Kodner2009). However, the wall in Branchinella appeared laminar in texture and without distinct layers, while the preparation artefacts obscured the Gyalosphaeridium wall section and made layers unrecognizable. A wall ultrastructure with a single homogeneous to composite four-layered structure differentiated by the texture and electron density of individual layers was observed in different specimens of Gyalosphaeridium pulchrum, as it is also known in algal cysts (Willman & Moczydłowska, Reference Willman and Moczydłowska2007; Moczydłowska & Willman, Reference Moczydłowska and Willman2009). In various extant microalgae, the number of layers and their texture changes through developmental phases of cyst formation in which additional layers are secreted as secondary wall layers during morphogenesis and cyst maturation (Hagen et al. Reference Hagen, Siegmund and Braune2002; Damiani et al. Reference Damiani, Leonardi, Pieroni and Cáceres2006). G. pulchrum was inferred to be the cyst of a chlorophycean green microalga (Moczydłowska & Willman, Reference Moczydłowska and Willman2009).
The surface morphology of the resting cyst in Branchinella observed in scanning electron microscope (SEM) images was also compared to that of Alicesphaeridium sp. (Cohen et al. Reference Cohen, Knoll and Kodner2009), and other unidentified acanthomorphic microfossils that were illustrated by LM images and were assumed to be morphologically analogous to animal cysts. As already stated, such complex ornamentation in cysts is known only in extant tardigrades and arthropods, which did not yet exist in the Ediacaran. Alicesphaeridium as well as Gyalosphaeridium were earlier suggested to be algal cysts (Grey, Reference Grey2005) and the subsequently proposed animal affinity of these taxa was questioned in subsequent studies (Moczydłowska et al. Reference Moczydłowska, Landing, Zang and Palacios2011; Moczydłowska & Nagovitsin, Reference Moczydłowska and Nagovitsin2012). The unidentified Ediacaran taxa illustrated by Cohen et al. (Reference Cohen, Knoll and Kodner2009, fig. 3d, e) are here assigned to Tanarium and respectively to Mengeosphaera (Cohen et al. Reference Cohen, Knoll and Kodner2009, fig. 3c) and studied here, further supporting their algal cyst recognition.
6.c. Previous interpretations of the Tianzhushania plexus affinities
Originally, the phosphatized microfossils discovered by Xue et al. (Reference Xue, Tang, Yu and Zhou1995) in the Weng’an locality and assigned to the new taxa Parapandorina, Megaclonophycus (later synonymized with Tianzhushania and Megasphaera; Xiao & Knoll, Reference Xiao and Knoll2000; Yin et al. Reference Yin, Bengtson and Yue2004; Xiao et al. Reference Xiao, Zhou, Liu, Wang and Yuan2014), and Spiralicellula were interpreted as belonging to the Class Chlorophyceae (orders Volvocales and Chlorococcales). This was based on clear observations of cell division into four, eight to multiple and hundreds of cells that were enclosed by the inferred mother cell or formed spheroidal colonies. Morphological similarity to extant volvocacean Pandorina Bory 1824, a cosmopolitan freshwater genus, prompted this affinity to be inferred for Parapandorina and Spiralicellula (Xue et al. Reference Xue, Tang, Yu and Zhou1995, Reference Xue, Zhou and Tang2001). Megaclonophycus was attributed by these authors to possible Chlorococcales because of its remarkable resemblance by having hundreds of cells densely filling the mother cell. The algal affinity of these taxa was later abandoned in favour of an animal embryo interpretation (Xiao et al. Reference Xiao, Zhang and Knoll1998; Xiao & Knoll, Reference Xiao and Knoll2000), but it deserves reconsideration given the arguments presented here for the affinity of Tianzhushania spinosa, and the recent arguments put forward for the biologically conspecific taxa Spiralicellula and Helicoforamina as chlorophycean green alga (Zhang & Pratt, Reference Zhang and Pratt2014; but see also Donoghue et al. Reference Donoghue, Cunningham, Dong, Bengtson and Wanninger2015). Helicoforamina, however, was suggested to be a holozoan without determining between the protistan or metazoan holozoan affinity (Yin et al. Reference Yin, Sun, Liu, Zhu and Donoghue2020).
The best-studied Tianzhushania plexus has been subsequently suggested to belong to holozoans (Huldtgren et al. Reference Huldtgren, Cunningham, Yin, Stampanoni, Marone, Donoghue and Bengtson2011; Donoghue et al. Reference Donoghue, Cunningham, Dong, Bengtson and Wanninger2015; Cunningham et al. Reference Cunningham, Vargas, Yin, Bengtson and Donoghue2017; but see Xiao et al. Reference Xiao, Knoll, Schiffbauer, Zhou and Yuan2012). However, in this hypothesis, the specimens of the Tianzhushania morphotype have not been examined and/or illustrated but instead its supposed developmental stages or synonyms: Megasphaera, Parapandorina, Megaclonophycus, Spiralicellula and peanut-shaped microfossils. This is because Tianzhushania cannot be recognized with any certainty in those studied phosphatized specimens, which do not preserve the diagnostic processes and outer membrane that are seen only in silicified specimens in thin-sections. Moreover, the here presented late ontogenetic stage with thousands of identical cells (Fig. 3f) contrasts with the differentiated cells of various sizes in peanut-shaped microfossils (see references above).
The newly recorded T. spinosa at advanced developmental stage disproves the animal affinity and demonstrates instead the algal character in addition to the vesicle morphology. This stage (Fig. 3f) or any other earlier stage (Fig. 3e) does not show the Megasphaera-type tuberculate or polygonal layer inside the process-bearing vesicle or any wavy outline of it and thus it is in question whether Megasphaera is a developmental morph of Tianzhushania. To the contrary, the internal cells within the spinose vesicle of T. spinosa are embraced by a thin, smooth membrane (Fig. 3e, left side, and other specimens not illustrated here; see also Shang et al. Reference Shang, Moczydłowska, Liu and Liu2018). We refrain from evaluating the synonyms or developmental morphs of T. spinosa, but we have reservations about such synonymy. Consequently, we consider Tianzhushania only to refer to the original morph of the type species T. spinosa. In some publications (Huldtgren et al. Reference Huldtgren, Cunningham, Yin, Stampanoni, Marone, Donoghue and Bengtson2011; Xiao et al. Reference Xiao, Knoll, Schiffbauer, Zhou and Yuan2012; Donoghue et al. Reference Donoghue, Cunningham, Dong, Bengtson and Wanninger2015; Cunningham et al. Reference Cunningham, Vargas, Yin, Bengtson and Donoghue2017), the inferred developmental morphs were attributed to Tianzhushania while illustrating in fact Megasphaera, Parapandorina or peanut-shaped microfossils and confusing the identification if such synonymy is not correct. We add new observations and comment on features evident in previously published specimens that are relevant to the discussion on alternative affinities of T. spinosa.
The features used to support the animal cyst and embryo interpretation were the large size of the cyst (400–1100 μm), 2 n pattern of cell division with decreasing size (= palintomic cell cleavage), Y-shaped cell junctions, geometry of the cell clusters, and ornate envelope (Xiao et al. Reference Xiao, Zhang and Knoll1998; Xiao & Knoll, Reference Xiao and Knoll2000; Xiao, Reference Xiao2002; CY Yin et al. Reference Yin, Bengtson and Yue2004; L Yin et al. Reference Yin, Zhu, Knoll, Yuan, Zhang and Hu2007). These features are also present in non-animal groups, such as the protistan holozoans (Lee et al. Reference Lee, Leedale and Bradbury2000; Butterfield, Reference Butterfield2011; Huldtgren et al. Reference Huldtgren, Cunningham, Yin, Stampanoni, Marone, Donoghue and Bengtson2011; Donoghue et al. Reference Donoghue, Cunningham, Dong, Bengtson and Wanninger2015), and all of them are equally applicable to algal cysts with reproducing cells (Van den Hoek et al. Reference Van den Hoek, Mann and Jahns1995; Raven et al. Reference Raven, Evert and Eichhorn2005; Lee, Reference Lee2008; Graham et al. Reference Graham, Graham and Wilcox2009). As argued above, the vesicle size is non-diagnostic and sizes of concerned microfossils are known in cysts of all organismal clades.
Binary fission (cell division of the 2 n pattern) is universal in all phylogenetic lineages, and palintomic division, which produces dyads, tetrads, octads, and further results in multiple cell clusters within ornamented vesicles, is characteristic of algal cysts (Raven et al. Reference Raven, Evert and Eichhorn2005; Lee, Reference Lee2008). The Y-shaped junction of cells is also found in algal reproductive tetrahedral cells and later-evolved embryophyte spores (Raven et al. Reference Raven, Evert and Eichhorn2005) and is not unique to tetrahedral geometry in animal early blastomeres. However, T-shaped junction and offset cells in algae, in contrast to the Y-shaped junction between dividing cells in microfossils that were argued to be animal embryos and in support of the recognition of microfossil affinities (Xiao, Reference Xiao2002), have been observed in multicellular algae in the same fossil assemblage and not in the unicellular algal cysts.
Polygonal or faceted individual cells that were interpreted to be blastomeres were only observed in the early cleaving stages of up to 16-cell ‘embryos’, but in the multi-celled stages the cells are spheroidal (Xiao et al. Reference Xiao, Zhang and Knoll1998; Xiao & Knoll, Reference Xiao and Knoll2000; Yin et al. Reference Yin, Zhu, Knoll, Yuan, Zhang and Hu2007, Reference Yin, Zhou and Yuan2008). In animal embryos, the blastomeres may be faceted, but in subsequently developing stages they change their shape when differentiating into layers and poles (Kessel & Shih, Reference Kessel and Shih1974; Mathews, Reference Mathews1986) and are not spheroidal, as observed in the microfossils. The dividing cells’ distortion in the microfossils that were argued to be animal embryos showed that they were not rigid in contrast to algal cell walls (Xiao, Reference Xiao2002). This comparison was made with multicellular algal vegetative cell walls and not the offspring cells within the cysts of unicellular algae. The dividing cells’ wall plasticity is not exclusively an animal feature. While algal cysts do have sturdy walls, reproducing algal cells are not rigid (Tappan, Reference Tappan1980; Evitt, Reference Evitt1985).
In phosphatized, 3D preserved specimens assigned to Tianzhushania (although not preserving its diagnostic processes) and its supposed synonyms from the Weng’an localities that were interpreted to be stereoblastulas (Xiao et al. Reference Xiao, Zhang and Knoll1998; Xiao & Knoll, Reference Xiao and Knoll2000; Xiao, Reference Xiao2002; Xiao et al. Reference Xiao, Knoll, Schiffbauer, Zhou and Yuan2012), the internal geometry of the cell clusters could not be observed, with the exception of Megaclonophycus in cross-section (Xiao & Knoll, Reference Xiao and Knoll2000). Thus, there is neither evidence of a stereoblastula (a blastula without a cavity but with differentiation of the inner and outer cells; or sterreblastula in Nielsen, Reference Nielsen2012) nor of a blastocoel. In silicified specimens from the Yichang area, the cross-section of Tianzhushania vesicles containing multiple cells (over 100; Yin et al. Reference Yin, Zhou and Yuan2008, pl. 1, figs 11, 13) shows identical spheroidal cells of the same size and tightly clustered without any central cavity, as in our record of T. spinosa from the Yangtze Gorges area comprising thousands of cells in the vesicle. In the cross-section of phosphatized Megaclonophycus, the spheroidal multiple cells (more than 100 or so; Xiao & Knoll, Reference Xiao and Knoll2000, figs 9:11, 9:12) show the same appearance of a dense cluster without a cavity. All these examples of mature developing stages of the cyst as well as the abundance and geometry of internal cells in Tianzhushania and Megaclonophycus are typical of an algal ornamented zygotic cyst or spheroidal smooth-walled cyst or mother cell, respectively, with multiple identical offspring cells.
There is no clear evidence of any intervening and morphologically dissimilar developmental stages or their transformation from T. spinosa based on observations of silicified specimens’ cross-sections. The previously suggested and morphologically different stages were studied in phosphatized specimens, and Tianzhushania was claimed to be among them but never recognized by diagnostic features. The idea that Tianzhushania lost the outer spinose wall and is preserved as Megasphaera and other morphs (Yin et al. Reference Yin, Bengtson and Yue2004) is not substantiated even when comparing the silicified and phosphatized Tianzhushania ornata vs Megasphaera ornata in cross-sections (Xiao & Knoll, Reference Xiao and Knoll2000; Yin et al. Reference Yin, Bengtson and Yue2004; Xiao et al. Reference Xiao, Knoll, Schiffbauer, Zhou and Yuan2012). Therefore, developmental stages must be confirmed, if truly existing, in cross-sections of silicified T. spinosa.
The holozoan interpretation of microfossils attributed to the Tianzhushania life cycle was based on the elegant documentation of subcellular morphology and bodies potentially identifying nuclei in Megasphaera, abundant cells preserved inside peanut-shaped specimens, and helical four cells in Spiralicellula by SEM and synchrotron X-ray microtomography (Huldtgren et al. Reference Huldtgren, Cunningham, Yin, Stampanoni, Marone, Donoghue and Bengtson2011; Donoghue et al. Reference Donoghue, Cunningham, Dong, Bengtson and Wanninger2015; Cunningham et al. Reference Cunningham, Vargas, Yin, Bengtson and Donoghue2017; see also Hagadorn et al. Reference Hagadorn, Xiao, Donoghue, Bengtson, Gostling, Pawlowska, Raff, Raff, Turner, Yin, Zhou, Yuan, McFeely, Stampanoni and Nealson2006). These studies demonstrated the lack of metazoan embryo synapomorphic features, but only in the above-mentioned synonymized microfossils and not directly for the Tianzhushania morphotype. The internal morphology of the peanut-shaped microfossil and the structures exuding from the Megasphaera vesicle were inferred to be germinating propagules with palintomically dividing cells inside, thus being synapomorphic to holozoan grade of organisms (Huldtgren et al. Reference Huldtgren, Cunningham, Yin, Stampanoni, Marone, Donoghue and Bengtson2011). As Butterfield (Reference Butterfield2011) pointed out, these developmental and cyst morphologic features may be considered among green microalgae by comparison with the chlorophycean Volvox life cycle, including zygote encysted and ornamented by processes.
The sequence of developmental stages in the life cycle of Tianzhushania was inferred to include the mother cell, Tianzhushania morphotype (or two mother cells, Tianzhushania and Spiralicellula), the encysted stage within the tuberculate envelope that is Megasphaera, and the stages with mitotic palintomic cleavage of Parapandorina, Megaclonophycus and the peanut-shaped microfossils (Huldtgren et al. Reference Huldtgren, Cunningham, Yin, Stampanoni, Marone, Donoghue and Bengtson2011; Donoghue et al. Reference Donoghue, Cunningham, Dong, Bengtson and Wanninger2015). In this reconstruction, it remains unclear how two morphologically distinct microfossils could be two mother cells, and there is no evidence of transformation between them, or between Tianzhushania and Megasphaera or to helical symmetry of four cells in Spiralicellula. Tianzhushania in an early stage containing a few cells has never shown any helical symmetry, and its morphology is diametrically different. The clear cell differentiation or so-called ‘individuality of cellular units toward the periphery’ observed in the peanut-shaped microfossil (Huldtgren et al. Reference Huldtgren, Cunningham, Yin, Stampanoni, Marone, Donoghue and Bengtson2011) is at odds with Tianzhushania spinosa containing thousands of identical cells observed herein, and we do not accept this morph as a stage in the life cycle of Tianzhushania or as of the same affinity. The peanut-shaped microfossils are not conspecific with Tianzhushania, which is not the mother cell but a reproducing cyst. The tuberculate or polygonal wall in Megasphaera and in the peanut-shaped microfossil is similar, but the shapes of these taxa are very different, and it is not certain why, if belonging to the same life cycle, the cyst would at one time germinate to release offspring cells (Megasphaera), and another time would be transformed to the peanut-shaped cyst and produce offspring cells different in shape. The ‘germinating tube’ exuding from Megasphaera can be compared with the release of the endocyst (membranous sack containing offspring cells) prior to the escape of offspring cells as seen in extant algae (Tappan, Reference Tappan1980; Dale, Reference Dale2001). Spiralicellula is not the synonym or developmental morph of Tianzhushania but is a distinct species (Zhang & Pratt, Reference Zhang and Pratt2014). The unnamed bilobate peanut-shaped specimens have been re-evaluated as not belonging to the same life cycle, neither being embryos of metazoans or holozoans nor their tuberculate surface sculpture being homologous to that of Megasphaera (Zhang & Pratt, Reference Zhang and Pratt2014). Their multiple cells (srXTM renderings; Huldtgren et al. Reference Huldtgren, Cunningham, Yin, Stampanoni, Marone, Donoghue and Bengtson2011, fig. 3g, j) also resemble multicellular algae (compare Yin et al. Reference Yin, Zhu, Tafforeau, Chen, Liu and Li2013; Ye et al. Reference Ye, Tong, An, Tian, Zhao and Zhu2015; Bengtson et al. Reference Bengtson, Sallsted, Belivanova and Whitehouse2017).
Taxa morphologically similar to the peanut-shaped or propagule-releasing microfossils, if not identical, and other unnamed taxa from the Weng’an phosphorites were examined by SEM and synchrotron X-ray microtomography by Yin et al. (Reference Yin, Zhu, Tafforeau, Chen, Liu and Li2013). These analyses revealed the presence of two internal cells with polar lobe stage, and equal and unequal cleavage stages inferred to be embryos. Exemplified by the sequence of cleavage stages of polar lobe-forming embryos in extant bilaterian animals, these Weng’an microfossils were taken as evidence for bilaterians existing at c. 580 Ma (Yin et al. Reference Yin, Zhu, Tafforeau, Chen, Liu and Li2013). Similar in overall shape, microfossils from the same assemblage but seen in thin-sections exhibit multiple cells in a pseudo-parenchymatous ‘cell fountain’ and true parenchymatous structures. These microfossils were inferred to be multicellular algae in which cells are of different shapes and sizes forming thallus with radial geometry (fountain) (Yin et al. Reference Yin, Zhu, Tafforeau, Chen, Liu and Li2013). Comparably, the peanut-shaped specimens with abundant cells of various shapes and linear alignment (Huldtgren et al. Reference Huldtgren, Cunningham, Yin, Stampanoni, Marone, Donoghue and Bengtson2011, fig. 3) may belong to multicellular algae, as interpreted by Yin et al. (Reference Yin, Zhu, Tafforeau, Chen, Liu and Li2013). Single cells (in surface layers) and oligocellular units (inside the microfossils) observed by Huldtgren et al. (Reference Huldtgren, Cunningham, Yin, Stampanoni, Marone, Donoghue and Bengtson2011) are differentiated in shape and distributed in various portions of the peanut-shaped microfossils and are unlike propagules in protists that would be of the same shape and size.
Phosphatized spheroidal microfossils from the Weng’an locality and attributed to Megaclonophycus stage and Megaclonophycus-like fossils, which show a palintomic cell division (dyads, tetrads and multiple cells within vesicle cavity) and purported ‘evidence for cell differentiation’, were reported by Chen et al. (Reference Chen, Xiao, Pang, Zhou and Yuan2014). These authors concluded that the fossils may represent stem-group animals or algae. In addition to multiple small spheroidal and polygonal cells, as seen in thin-sections within the vesicle cavity, the structures called matryoshkas (derivative name from Russian nesting dolls of same shape but decreasing sizes) were observed. These structures are of variable sizes in a wide range and multicellular themselves but not following a palintomic cell division (thus rather not algal) and were interpreted as growing structures with ‘cytoplasmic growth after each division to restore cell size’ (Chen et al. Reference Chen, Xiao, Pang, Zhou and Yuan2014). Megaclonophycus was further suggested to show cell-to-cell adhesion, multicellularity, cell differentiation in peripherial layer, germ–soma separation (matryoshkas being germ cell structures), and to represent ‘stem-group animals that evolved an autapomorphic life cycle involving a matryoshka stage’ (Chen et al. Reference Chen, Xiao, Pang, Zhou and Yuan2014). The matryoshka structures were, however, argued not to pertain to the developmental cycle of Megasphaera–Parapandorina–Megaclonophycus and could be parasitic or symbiotic organisms (Tang, Reference Tang2016). These structures are present in only a few individuals and if representing germ cells should be seen in most or all mature individuals of Megaclonophycus, the morphotype with abundant monads, dyads and tetrads, but sporadic matryoshkas. Matryoshka structures grew larger with increasing cell number, proving non-palintomic cell divisions (Tang, Reference Tang2016).
The alleged cell differentiation in size, shape and arrangement in peripheral layer of Megaclonophycus stage (Chen et al. Reference Chen, Xiao, Pang, Zhou and Yuan2014, fig. 1d–f) as distinct from loosely aggregated cells infilling the vesicle cavity (Chen et al. Reference Chen, Xiao, Pang, Zhou and Yuan2014, fig. 1g, h) is not demonstrated. The cells are spheroidal, attached or not, or packed and distorted to polygonal shape, but not consistently in a layer. These features are likely taphonomic and show disintegration of cells, like some other cells in the central portion of the vesicle, and are intercalated with small voids that are permineralized by cement (Chen et al. Reference Chen, Xiao, Pang, Zhou and Yuan2014, fig. 1f, periphery of vesicle; fig. 1d, e, in a mass of cells). Specimens enclosing matryoshkas are not even recognizable with certainty as Megaclonophycus (Chen et al. Reference Chen, Xiao, Pang, Zhou and Yuan2014, fig. 3). Matryoshka with envelope bearing branching protrusions or ‘ornamentation’ (Chen et al. Reference Chen, Xiao, Pang, Zhou and Yuan2014, fig. 3b, c) is not of the same shape as the enclosing Megaclonophycus’s vesicle envelope that is supposedly tubercular or ornamented by conical elements. The envelope protrusions are irregular tubular, hollow and connected by their interiors with the matryoshka’s cavity containing disintegrated remnants of cells (Chen et al. Reference Chen, Xiao, Pang, Zhou and Yuan2014, fig. 3c). Protrusions are of the same appearance as the reticulate meshwork of organic material infilling the vesicle cavity of Megaclonophycus (Chen et al. Reference Chen, Xiao, Pang, Zhou and Yuan2014, fig. 3b) and could be fungal hyphae or diagenetic modifications and taphonomic remnants of degraded organic matter. Similar in morphology are fungal zygosporangia, sporocarps or hyphae (cf. Taylor et al. Reference Taylor, Krings and Taylor2015, figs 6.27, 7.13, 8.62, 9.16). Fungal hyphae can form meshwork (Taylor et al. Reference Taylor, Krings and Taylor2015, fig. 8.9), which resembles the pattern seen in the partly destroyed and remineralized cavity of Megaclonophycus-like fossil beside of matryoshka (Chen et al. Reference Chen, Xiao, Pang, Zhou and Yuan2014, fig. 3b, c). This matryoshka is more likely fungal overgrowth. Another matryoshka structure illustrated (Chen et al. Reference Chen, Xiao, Pang, Zhou and Yuan2014, fig. 3i, j) does not belong to Megaclonophycus-like fossil but it superimposed on its surface and mostly disintegrated into a clump of organic material. Taphonomic artefacts resembling pseudo-processes are also seen in other microfossils affected by degradation (cf. Liu et al. Reference Liu, Xiao, Yin, Chen, Zhou and Li2014, figs 6:4, 6:6, 8:1, 19:1, 29:1; also comments by Hagadorn et al. Reference Hagadorn, Xiao, Donoghue, Bengtson, Gostling, Pawlowska, Raff, Raff, Turner, Yin, Zhou, Yuan, McFeely, Stampanoni and Nealson2006 and Donoghue et al. Reference Donoghue, Cunningham, Dong, Bengtson and Wanninger2015). In any case, the matryoshka structures are neither convincing germ cell structures nor does Megaclonophycus demonstrate cell differentiation as claimed by Chen et al. (Reference Chen, Xiao, Pang, Zhou and Yuan2014). Megaclonophycus is more likely an algal cyst with multiple spores inside the cavity.
As discussed, our interpretation is that Tianzhushania spinosa is neither a metazoan embryo nor a holozoan protist but an algal cyst. The record of unicellular algae among the suggested chlorophytes just c. 2 Ma after the end-Cryogenian deglaciation (Appendisphaera and Tianzhushania) is not a surprise because their basal lineages have evolved much earlier and persisted throughout the geological ages, surviving the Cryogenian ice ages into the Ediacaran (Moczydłowska, Reference Moczydłowska2008 a, b). The record of multicellular algae in the Ediacaran (the Lantian and Miaohe biotas and others; Steiner, Reference Steiner1994; Xiao et al. Reference Xiao, Zhang and Knoll1998, Reference Xiao, Knoll, Zhang and Hua1999; Xiao, Reference Xiao2002, Reference Xiao2004; Xiao et al. Reference Xiao, Knoll, Yuan and Pueschel2004; Yuan et al. Reference Yuan, Xiao, Yin, Knoll, Zhou and Mu2002, Reference Yuan, Chen, Xiao, Zhou and Hua2011; Ye et al. Reference Ye, Tong, An, Hu, Tian, Guan and Xiao2019) shows the increasing diversity of photosynthesizing organisms in a species-rich, expanding holomarine ecosystem.
7. Conclusions
The Ediacaran microfossils studied here, including seven species of Appendisphaera, Mengeosphara, Tanarium, Urasphaera and Tianzhushania, are inferred to represent algal zygotic cysts of unicellular chlorophytes (green algae). This interpretation is better supported now than previously suggested for Appendisphaera and Tanarium, and for the first time proposed for Tianzhushania spinosa, Mengeosphara and Urasphaera, by the new evidence of late ontogenetic stages containing multiple cells in clusters without any free cavity or cell differentiation.
The studied microfossils are composed of refractory biopolymers and contain multiple cleaving and identical offspring cells. They show the overall body plan and individual morphologic elements of cysts, as well as palintomic cell division and geometry of cell clusters in the process resembling sporogenesis that are known in extant green algae.
The life cycle of Tianzhushania spinosa was simple and confined to this cyst morphotype in which an enclosed, smooth-walled membranous maturing endocyst contained the zygote that palintomically divided into identical and abundant offspring cells. There is no evidence of intermediate developmental stages with different morphologies between T. spinosa with a few cells and the same morph with thousands of cells. Morphologically dissimilar and conspicuous microfossils previously interpreted to represent developmental stages in the Tianzhushania life cycle are considered not to be conspecific and its ontogenetically changing morphs. The peanut-shaped microfossils with abundant and differentiated cells contrast with the late stage of Tianzhushania that has exclusively identical cells, and their suggested holozoan affinity remains uncertain because of these cells’ differentiation pattern.
The lack of any free cavity within tightly packed clusters of cells within the cyst precludes the comparison with blasto- or gastrocoel, and the lack of cell differentiation or layering that would be expected in animal embryos dismisses the animal affinity for all studied microfossil taxa.
The question remains, did animals appear at the very beginning of the Ediacaran and produce resistant reproductive cysts of morphological complexity such as among the recorded microfossils, although adult animals are not preserved? So far, there is no conclusive evidence for such a supposition. The cnidarian grade of metazoans recognized at this time among the Lantian biota definitely has no such complex life history.
Acknowledgements
Our research was supported by the Swedish Research Council (Vetenskåpsrådet) project grant Nr 621-2012-1669 to M.M., and the National Natural Science Foundation of China (41572016), China Geological Survey (121201102000150010-06) and National Key Research and Development Program of China (2016YFC0601001) to P.L. Marien van Westen (University of Groningen, the Netherlands) kindly provided the images of extant zygotic cysts in Figure 4a–b.
Author contributions
M.M. conceived and designed the research. P.L. collected the material and photographed specimens. M.M. and P.L. conducted microscopy, developed the interpretation and prepared the manuscript.
Declaration of interests
The authors declare no competing interests.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0016756820001405







