1. Introduction
A good model in which to study chromosomal speciation is the house mouse (Mus musculus), since much information regarding both its genome constitution and the diversity of its chromosomal races is available (Dietrich et al., Reference Dietrich, Miller, Steen, Merchant, Damron-Boles, Husain, Dredge, Daly, Ingalls and O'Connor1996; Mouse Genome Sequencing Consortium, 2002; Piálek et al., Reference Piálek, Hauffe and Searle2005). The standard karyotype of M. musculus has 40 acrocentric chromosomes (19 pairs of autosomes and the sex chromosomes); nevertheless, wild populations of the western European house mouse (M. musculus domesticus) show high karyotype diversification due to Robertsonian (Rb) fusions, i.e. centric fusion between two non-homologous acrocentric chromosomes to form a metacentric. This diversification seems to have been occurring in the 3000 years since the arrival of house mouse in Western Europe (Cucchi et al., Reference Cucchi, Vigne and Auffray2005). Although the geographic distribution of the house mouse includes much of the world, these centric fusions have been reported only in some populations from the Orkney Islands (Scotland) to the North African coast and the Middle East, and they produce karyotypes with diploid numbers ranging from 22 to 39 (for review see Piálek et al., Reference Piálek, Hauffe and Searle2005). Up to now 97 metacentric populations have been recorded and, among all the 171 possible acrocentric combinations, 102 have been reported in wild populations (Piálek et al., Reference Piálek, Hauffe and Searle2005; Sans-Fuentes et al., Reference Sans-Fuentes, López-Fuster, Ventura, Díez-Noguera and Cambras2005). The high Rb chromosomal variation in the house mouse can be explained by high mutation and/or high fixation rates (see White, Reference White1978; Winking, Reference Winking1986; Capanna & Redi, Reference Capanna and Redi1995; Nachman & Searle, Reference Nachman and Searle1995).
Chromosomal polymorphism in the vicinity of Barcelona is found in one of the well-characterized regions containing Rb populations. This area was first described by Adolph & Klein (Reference Adolph and Klein1981), who reported the fusions Rb(4.14), Rb(5.15), Rb(9.11), Rb(12.13) and Rb(6.10), and animals with a diploid number ranging between 30 and 40. Later, Gündüz et al. (Reference Gündüz, López-Fuster, Ventura and Searle2001) described a new Rb translocation in this area [Rb(3.8)], and hypothesized the presence of a new race (as defined by Hausser et al., Reference Hausser, Fedyk, Fredga, Searle, Volobouev, Wojcik and Zima1994) with chromosomes Rb(3.8), Rb(4.14), Rb(5.15), Rb(6.10), Rb(9.11) and Rb(12.13) in the homozygous state and with a diploid number of 28. The main characteristics of this area are: (i) it is geographically widespread (5000 km2), (ii) staggered clines for Rb chromosome have been described (Gündüz et al., Reference Gündüz, López-Fuster, Ventura and Searle2001) and (iii) until now, no chromosomal race has been found. Our surveys in this geographic region revealed a new fusion, Rb(7.17), whose presence has been mentioned in previous studies (Muñoz-Muñoz et al., Reference Muñoz-Muñoz, Sans-Fuentes, López-Fuster and Ventura2003, Reference Muñoz-Muñoz, Sans-Fuentes, López-Fuster and Ventura2006; Sans-Fuentes et al., Reference Sans-Fuentes, López-Fuster, Ventura, Díez-Noguera and Cambras2005) but the geographic distribution and frequency of which have never been reported. This fusion is of special interest for several reasons: (i) two chromosomes of very different sizes are involved; (ii) chromosome 7 has been found fused with only a few other chromosomes (Piálek et al., Reference Piálek, Hauffe and Searle2005); (iii) chromosome 7 is rich in imprinted genes (Coan et al., Reference Coan, Burton and Ferguson-Smith2005); and (iv) chromosome 17 harbours the t-complex, related to the impairment of fertility in mice (Lyon, Reference Lyon1991; Ardlie, Reference Ardlie1998). Here we report data on the distribution and frequency of this fusion.
2. Materials and methods
In this study we report data for 134 mice trapped between 1998 and 2002 in four localities from the Barcelona polymorphic Rb area: Garraf, Vilanova i la Geltrú, La Granada and Calafell (Fig. 1). This sample is part of a wider study that is being conducted in the Barcelona Rb polymorphic area, and corresponds to the sites where animals with Rb(7.17) were detected. Data reported by previous studies in this area for these localities were also included in the analyses (n=23; table 1 in Gündüz et al., Reference Gündüz, López-Fuster, Ventura and Searle2001). It is worth mentioning that in this previous study Rb(7.17) was not detected.
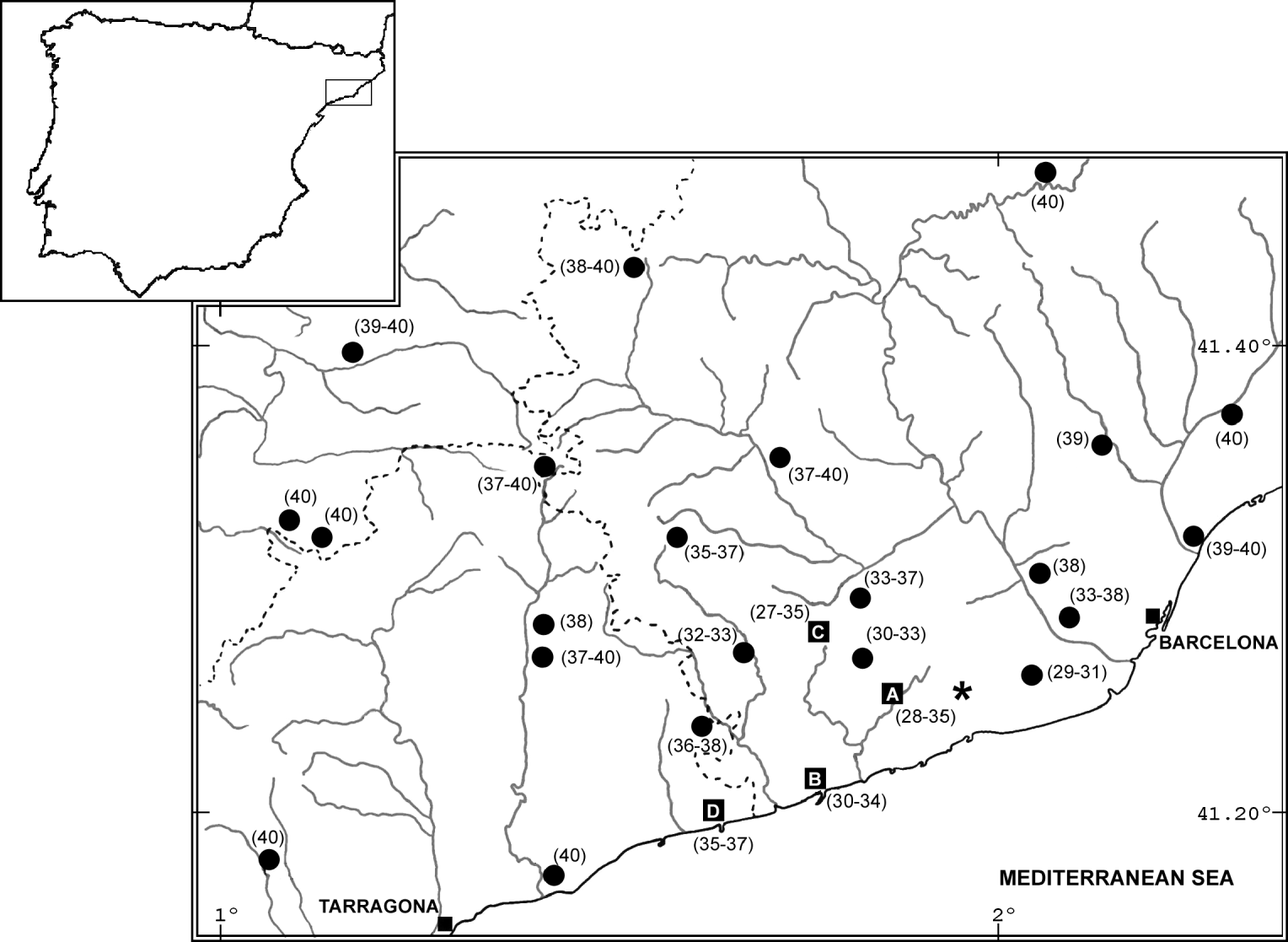
Fig. 1. Known geographic area of the Barcelona Robertsonian polymorphism zone of M. m. domesticus. Localities sampled for this study are labelled by a letter (for the names see Table 1). The localities sampled in previous studies are shown by circles (for details see Gündüz et al., Reference Gündüz, López-Fuster, Ventura and Searle2001). The diploid number range for each locality is shown in parentheses. The star indicates the centre of Barcelona Rb Polymorphism area according to Gündüz et al. (Reference Gündüz, López-Fuster, Ventura and Searle2001).
Metaphase karyotypes of mice were prepared following Ford (Reference Ford1966). Slides with spreads were incubated for 12 h at 37°C. After this period, metaphases were stained using Wright staining for G bands (Mandahl, Reference Mandahl1992). On the basis of the analysis of 10 metaphase spreads per animal, chromosomes were identified, following the Committee on Standardized Genetic Nomenclature for Mice (1972).
Since some localities were sampled over several years (La Granada in 1998, 1999, 2000, 2002; Garraf in 1998, 1999), Fst (Wright's fixation index) was calculated for these sites to measure temporal differentiation (Weir & Cockerham, Reference Weir and Cockerham1984). To test whether Fst values differed significantly from zero, 10 000 genotype permutations between samples were performed. This is a powerful method for detecting temporal differences among populations (Goudet et al., Reference Goudet, Raymond, de Meeüs and Rousset1996; Lugon-Moulin et al., Reference Lugon-Moulin, Brünner, Wyttenbach, Hausser and Goudet1999). We postulate that temporal differentiation was not found, thus samples from the same locality were pooled in subsequent analyses. These analyses were obtained with FSTAT v2.9.3.2 (Goudet, Reference Goudet1995, Reference Goudet2001).
Mean diploid number was calculated for each locality. Metacentric frequencies and mean heterozygosity were also calculated for each locality using BIOSYS-2 (updated version of BIOSYS-1; Swofford & Selander, Reference Swofford and Selander1981). To evaluate deviations from Hardy–Weinberg (H-W) equilibrium of each metacentric, an exact probability test was performed for each sample. This test avoids the difficulties of the χ2 distribution for small samples (Haldane, Reference Haldane1954; Guo & Thompson, Reference Guo and Thompson1992). The P value was calculated using the complete enumeration method described by Louis & Dempster (Reference Louis and Dempster1987). H-W equilibrium deviations for the set of all metacentrics in each sample were studied by the Fisher method (Raymond & Rousset, Reference Raymond and Rousset1995). When multiple tests were used in an analysis, significance levels were adjusted by Bonferroni sequential correction (Rice, Reference Rice1989). All these tests were performed on minimum samples of five mice using GENEPOP v3.4 (Raymond & Rousset, Reference Raymond and Rousset1995).
It has been pointed out by some authors that the transmission rate in progeny is related to both total length and arm length ratio of the Rb chromosomes (White, Reference White1973; King, Reference King1993; Castiglia & Capanna, Reference Castiglia and Capanna2000). Therefore, for each metacentric, both length and arm length ratio were estimated. For this purpose we used the chromosome lengths expressed as a percentage of the haploid female complement provided by the Committee on Standardized Genetic Nomenclature for Mice (1972). Total length was estimated as the sum of the lengths of each acrocentric, and the arm length ratio was estimated as the percentage of the shorter arm relative to the longer one. The latter is a measure of the difference in size between the acrocentrics involved in an Rb fusion. For example, an arm length ratio of 50% for Rb(a.b), means that arm ‘b’ is half the size of arm ‘a’. An arm length ratio of 100% indicates that the two arms are the same size.
3. Results and discussion
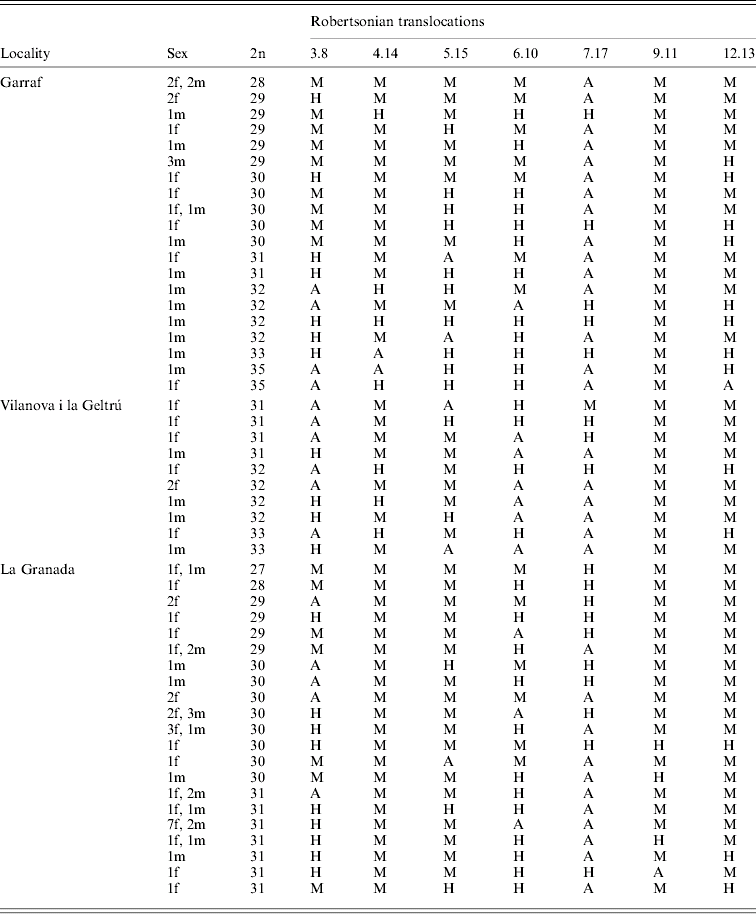
Of the 134 animals karyotyped, we obtained full data on 120 mice (Fig. 1, Appendix). The remaining 14 animals provided only information on the diploid number (see Table 1 for details). No deviations from H-W equilibrium expectations were detected. The chromosome number of animals from these sites ranged from 27 to 37 (Fig. 1, Appendix). The animals with the lowest diploid number (2n=27) were found in La Granada (C in Fig. 1), and carried the fusions Rb(3.8), Rb(4.14), Rb(5.15), Rb(6.10), Rb(7.17), Rb(9.11) and Rb(12.13) (Fig. 2). The metacentric with the lowest frequency was Rb(7.17), with values ranging between 0·02 and 0·14 (Table 1). No other metacentric in the Barcelona Rb polymorphic area with such a low frequency range has been reported (table 1 in Gündüz et al., Reference Gündüz, López-Fuster, Ventura and Searle2001). Moreover, Rb(7.17) was distributed in a restricted area. The fusion Rb(7.17) was detected in four localities up to 32 km from the centre of the polymorphism area, located by Gündüz et al. (Reference Gündüz, López-Fuster, Ventura and Searle2001) between Garraf and Viladecans (Fig. 1). Although the fusions Rb(3.8) and Rb(6.10) also showed a small geographic distribution in this area, their frequencies were higher than those of Rb(7.17), both reaching values of 0·7. The fusions Rb(4.14), Rb(5.15), Rb(9.11) and Rb(12.13) presented a wider distribution (for details see table 1 in Gündüz et al., Reference Gündüz, López-Fuster, Ventura and Searle2001).

Fig. 2. G-banding staining of metaphase chromosomes of bone marrow cell from a specimen with 2n=27. All the Rb fusions are shown.
Table 1. Collection sites corresponding to localities where mice with Rb(7.17) have been captured and its chromosomal characteristics

For sample size (N), bold numbers correspond to data from Gündüz et al. (Reference Gündüz, López-Fuster, Ventura and Searle2001). Numbers in parentheses indicate animals for which only diploid number is known; these specimens were used only to calculate mean 2n. H, mean number of heterozygote metacentrics per individual.
a The number in parentheses corresponds to 1 animal with 2n=28, 3 with 2n=29, 3 with 2n=30, 3 with 2n=31, 1 with 2n=32 and 1 with 2n=33.
b The number in parentheses corresponds to 1 animal with 2n=30, 1 animal with 2n=31 and 2 animals with 2n=32.
c The number in parentheses corresponds to 2 animals with 2n=32.
These new samples complement the data reported by Gündüz et al. (Reference Gündüz, López-Fuster, Ventura and Searle2001) and suggest the lack of a chromosomal race in the Rb polymorphism zone of Barcelona. The combination of the specific Rb metacentrics observed near Barcelona has not been reported in any other population of M. m. domesticus (for review see Piálek et al., Reference Piálek, Hauffe and Searle2005). Specifically, fusions Rb(7.17), Rb(9.11) and Rb(12.13) have been described only in this area, and therefore this polymorphism is likely to have a partially independent origin. Nevertheless we can not exclude the fact that the others fusions [Rb(3.8), Rb(4.14), Rb(5.15), Rb(6.10)] could have been introduced.
Three of the four localities shown in this study were previously sampled by Gündüz et al. (Reference Gündüz, López-Fuster, Ventura and Searle2001) with no report of Rb(7.17). This could be due to the sample size for these sites (table 1 in Gündüz et al., Reference Gündüz, López-Fuster, Ventura and Searle2001). Although the restricted distribution of this mutation observed from these new data could be attributable to a biased sampling, it does not seem likely. The sample size of localities analysed here is higher than that in previous studies, and the re-sampling of some of the surroundings localities did not reveal the presence of this mutation (data not shown).
The information available does not allow us to give an accurate explanation of the restricted geographic distribution and low frequency of Rb(7.17), but it is possible that it is the result of the combined action of several factors, such as a recent mutation event and a transmission ratio distortion (TRD) due to the specific characteristics of this metacentric. As for the first concern, data available on laboratory strains suggest that Rb translocations appear spontaneously at high frequency (Nachman & Searle, Reference Nachman and Searle1995). Although this hypothesis seems the most plausible, forces acting against fixation can not be excluded a priori. Morphological characteristics might be important in the distribution and frequency of the fusions. Thus, the length of the metacentrics has been claimed as possible cause of TRD. For example, Castiglia & Capanna (Reference Castiglia and Capanna2000) found a higher transmission rate in the progeny for smaller metacentrics in heterozygous hybrids between Ciuttaducale (2n=22) and standard races (2n=40) in the centre of Italy. The total lengths of the metacentrics of the Robertsonian polymorphic area of Barcelona were: Rb(3.8), Lm. 11·10%; Rb(4.14), Lm. 10·20%; Rb(5.15), Lm. 9·59%; Rb(6.10), Lm. 10·45%; Rb(7.17), Lm. 9·17%; Rb(9.11), Lm. 9·01%; Rb(12.13), Lm. 9·97%. Taking into account these values and the frequency of distribution of the metacentrics in this area (see also Gündüz et al., Reference Gündüz, López-Fuster, Ventura and Searle2001), it seems that in this polymorphic zone the total length of these chromosomes is not directly related to their transmission rate. On the other hand, a heterozygous metacentric formed by two chromosomes of very different size may be affected by spatial orientation impairment during meiosis, thereby increasing the probability of malsegregation (White, Reference White1973; King, Reference King1993). The arm length ratios for the metacentrics of the Robertsonian polymorphic area of Barcelona were: Rb(3.8), 81·97%; Rb(4.14), 73·17%; Rb(5.15), 71·90%; Rb(6.10), 85·94%; Rb(7.17), 68·88%; Rb(9.11), 93·35%; Rb(12.13), 94·60%. Since Rb(7.17) is the metacentric in which the two contributing acrocentrics differ most in size (lowest arm length ratio), the arm length ratio may contribute to the low frequency and uncommon occurrence of Rb(7.17).
Other causes related to the low frequency of this fusion could be the genomic characteristics of the chromosomes involved in Rb(7.17). Mouse chromosome 7 is rich in imprinted genes (Coan et al., Reference Coan, Burton and Ferguson-Smith2005). Some authors have suggested the possibility that some of these genes may fail to be expressed properly in the Rb chromosomes, affecting their segregation (for details see Underkoffler et al., Reference Underkoffler, Mitchell, Abdulali, Collins and Oakey2005). This could be the reason why chromosome 7 has been found fused with only a few different chromosomes (Piálek et al., Reference Piálek, Hauffe and Searle2005), and why Rb(7.17) occurs in low frequency in Barcelona polymorphic area. Additionally, t-haplotypes, which are variants of chromosome 17 consisting of four non-overlapping inversions in the proximal third of this chromosome, are frequent in house mice and result in different degrees of sterility and often embryonic lethality of both sexes (Hammer et al., Reference Hammer, Schimenti and Silver1989; Lyon, Reference Lyon1991). Complete t-haplotypes have been detected in standard mice in two localities close to Barcelona (Silver et al., Reference Silver, Hammer, Fox, Garrels, Bucan, Herrmann, Frischauf, Lehrach, Winking, Figueroa and Klein1987), and partial t-haplotypes in mice from Barcelona city, located in the Rb polymorphic area (Figueroa et al., Reference Figueroa, Neufeld, Rittet and Klein1988). Since Rb fusions show a reduction of recombination in the pericentromeric area (Davisson & Akeson, Reference Davisson and Akeson1993), the presence of a partial t-haplotype linked to the fusion Rb(7.17) is likely. If so, both the limited geographic distribution of Rb(7.17) and the predominance of the heterozygous state for this fusion (only one animal showed the translocation in a homozygous state) could be indicative of the presence of a partial t-haplotype linked to this fusion, resulting in the low frequencies for this metacentric. In the light of the available information on the Robertsonian polymorphic area of Barcelona, further studies, both mating and molecular, are needed to investigate these hypotheses.
Special thanks go to Dr Michael Nachman, Dr Jeremy Searle, Dr Patrick Basset and Tovah Salcedo, as well as to the editor and two anonymous reviewers for their corrections and suggestions on the manuscript. This study was supported by a grant from the Spanish Ministerio de Ciencia y Tecnología (BMC2000-0541), and by a FPI grant from the Spanish Ministerio de Educación y Ciencia.
4. Appendix. Karyotype characteristics for all mice trapped between 1998 and 2002 in the four localities studied
Location, sex (f, female; m, male), diploid number (2n) and metacentric state (M, homozygote metacentric; H, heterozygote metacentric; A, homozygote acrocentric) are reported.