1. Introduction
Evolutionary and ecologically important aspects of variability and adaptation of fungi are highly associated with their mode of reproduction. Fungi are known to display diverse reproduction patterns (Taylor et al., Reference Taylor, Geiser, Burt and Koufopanou1999; Dyer, Reference Dyer2008). There are two major kinds of reproductive patterns that differ in principle: sexual (by means of meiospores) and asexual or clonal (by means of mitospores). These reproductive modes perform different functions in the life cycle of a fungus and are considered to have their own advantages and disadvantages as evolutionary strategies. Sex is regarded as an expensive process because the amount of offspring for one sexual parent is less than for one clonal parent, although for fungi, the actual cost of sex is much lower compared to animals and plants (e.g. Aanen & Hoekstra, Reference Aanen, Hoekstra, Heitman, Kronstad, Taylor and Casselton2007). It is postulated that sexual reproduction serves as a ‘conservative’ mechanism preserving the genome from degradation by facilitating selection against harmful mutations, and as a factor of genome flexibility that increases the probability of survival in a competitive and/or changing environment and expedites the appearance of evolutionary innovations (Maynard Smith, Reference Maynard Smith1978; Elliot, Reference Elliot1994; Korol et al., Reference Korol, Preygel and Preygel1994; Otto & Gerstein, Reference Otto and Gerstein2006; Goddard, Reference Goddard, Heitman, Kronstad, Taylor and Casselton2007).
On the other hand, asexual propagation is believed to be a successful evolutionary strategy for well-adapted genotypes in a stable, even if extreme, environment (Murtagh et al., Reference Murtagh, Dyer and Crittenden2000). About 20% of known fungal species are considered to be asexual (Dyer & Paoletti, Reference Dyer and Paoletti2005). For clonal fungi, there is a potential for mitotic recombination in heterokaryons via a parasexual cycle, which may be an important mechanism for diversification within a taxon (e.g. Debetes, Reference Debetes, Bridge, Couteaudier and Clarkson1998). Recent studies have shown that some morphologically asexual parasitic and saprotrophic species in fact possess a sexual cycle (Braumann et al., 2008, and references therein). The sexual phase may be either cryptic or rare, occurring only under very special conditions hardly observable in laboratory cultures. One such case is Aspergillus fumigatus, for which a fully functional sexual reproduction cycle leading to the production of heterothallic teleomorph Neosartorya fumigata was recently discovered (O'Gorman et al., Reference O'Gorman, Fuller and Dyer2009). Thus, for some fungal species, the sexual states may have been overlooked for a long period of time. Due to the diversity of reproductive strategies, fungal species represent an ideal target for experimental studies of the evolution of sex, to fill the gap between the ever-increasing spectrum of theoretical models and scenarios along with very limited empirical evidence for nearly every important characteristic on which these models are based.
The ascomycete Emericella (Aspergillus) nidulans (Eidam) Vuill. is a fungus that in culture easily generates two morphologically distinct kinds of sporulation: sexual (teleomorphic state, producing ascospores) and asexual (anamorphic Aspergillus state, producing conidia). Both spore types can be produced via clonal (selfing for ascospores and asexual cycle for conidia) and recombinant (outcrossing for ascospores and parasexual cycle for conidia) modes of reproduction (Pontecorvo, Reference Pontecorvo1956). Historically, E. nidulans was the first fungus for which the parasexual cycle was described (Pontecorvo et al., Reference Pontecorvo, Roper, Hemmons, MacDonald and Bufton1953). The fungus is homothallic (i.e. self-compatible or self-fertile) and, as a rule, haploid, but it can also exist as a heterokaryon and as a diploid. E. nidulans is widely distributed in different climatic and geographic areas across the world (Domsch et al., Reference Domsch, Gams and Anderson2007), including Israel (Volz et al., Reference Volz, Ellanskaya, Grishkan, Wasser and Nevo2001).
Previously, we studied the genetic variation of Israeli populations of E. nidulans on regional and local scales using seven microsatellite (simple sequence repeats or SSR) markers (Hosid et al., Reference Hosid, Grishkan, Frenkel, Wasser, Nevo and Korol2008). The study was performed in the framework of the ‘Evolution Canyon’ research programme at the Institute of Evolution, University of Haifa, focusing on the effect of microscale environmental variability on biodiversity patterns (Nevo, Reference Nevo2001) in three ‘Evolution Canyons’ (ECs). The first two canyons (EC I and EC II) are located in the northern part of Israel at a distance of 38 km from each other. The desert EC III is located southward at a distance of nearly 350 km from the northern ECs. In each canyon, E. nidulans strains were isolated from the opposite slopes (in EC III also from the valley bottom (VB)). All three EC populations of E. nidulans were found to be genetically distinct from one another. The estimated genetic differentiation corresponds to geographical and ecological differences among the three microsites. On a regional scale, SSR polymorphism tended to increase with the severity of ecological conditions.
Our present study, based on 15 microsatellite markers, focuses on testing the reproductive, sexual versus clonal structure of the aforementioned E. nidulans populations. The population reproductive structure can be assessed by measuring correlated genetic diversity displayed by alleles at different loci and by comparison of phylogenetic trees constructed using molecular variation at each locus (reviewed in Taylor et al., Reference Taylor, Geiser, Burt and Koufopanou1999). In this paper, we demonstrate that the reproductive mode in E. nidulans may be population-specific: occurrence of sexuality was found in the northern populations and predominant clonality was found in the desert population.
2. Material and methods
(i) Sites of study
The study was conducted in three ECs: Lower Nahal Oren, Mt. Carmel (EC I, 32°43′N, 34°58′E); Lower Nahal Keziv, western Upper Galilee (EC II, 33°02′N, 35°11′E) and Nahal Shaharut, the southern Negev desert (EC III, 29°55′N, 34°58′E). In each canyon, samples were taken from the two opposite slopes – north-facing slope (NFS) and south-facing slope (SFS), separated by 50–150 m at the VB. EC I and EC II, located in the northern part of Israel, are characterized by sharply different microclimatic conditions (Pavlicek et al., Reference Pavlicek, Sharon, Kravchenko, Saaroni and Nevo2003). Different plant communities have developed on the opposite slopes: garrigue or savannoid, open park forest on the south-facing ‘African’ slopes and dense forest on the north-facing ‘European’ slopes. EC III represents an extreme desert location, with very sparse shrub vegetation growing only on the NFS and VB. For a detailed description of the canyons, see Hosid et al. (Reference Hosid, Grishkan, Frenkel, Wasser, Nevo and Korol2008).
(ii) Soil sampling and isolation of strains
Soil samples were taken from the upper layer (1–3 cm deep) of the SFS and NFS at EC I and EC II, and from the NFS and VB at EC III. Altogether, 175 strains of E. nidulans were isolated: 43 from EC I (18 and 25 from the SFS and NFS, respectively, in 2002), 55 from EC II (29 and 26 from the SFS and NFS, respectively, also in 2002) and 77 from EC III (61 from the VB, 16 from the NFS, in 2004). The strains were isolated by the soil dilution plate method (Davet & Rouxel, Reference Davet and Rouxel2000) using dilutions of 1:10, 1:100 and 1:1000 for soil:sterile water. The strains were identified using cultural and morphological criteria (Klich, Reference Klich2002). The isolated strains are preserved at 4°C in the culture collection of the Institute of Evolution, University of Haifa.
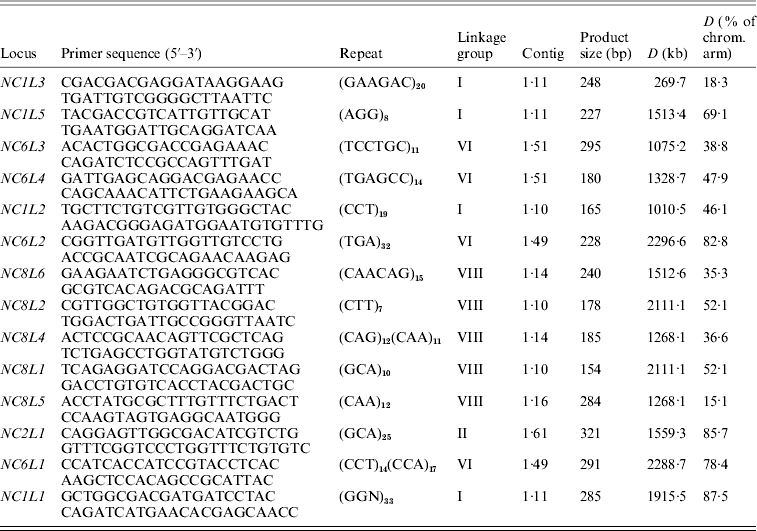
Growth of the strains, DNA isolation, PCR amplification and statistical analysis were performed as it was described in our previous work (Hosid et al., Reference Hosid, Grishkan, Frenkel, Nevo and Korol2005, Reference Hosid, Grishkan, Frenkel, Wasser, Nevo and Korol2008). The Spearman rank correlation test (StatSoft, 1996) was employed to estimate correlation between the variability of SSR markers (variance in repeat number) and physical distance from the markers to the centromere (% of chromosome arm length) (Table 1).
Table 1. Description of SSR markers of E. nidulans
(iii) Mode of reproduction of E. nidulans populations
Mode of reproduction of E. nidulans populations was tested using two methods for mutual control of the results obtained: the index of association (IA) and linkage disequilibrium (LD).
(1) The IA was calculated using the MultiLoc program (http://www-bs.informatik.uni-tuebingen.de/Services/MultiLoc). This index quantifies the amount of recombination among a set of sequences and detects association between alleles at different loci (Maynard Smith et al., Reference Maynard Smith, Smith, O'Rourke and Spratt1993). In populations with frequent recombination events, the value of IA is expected to be zero. Clonal populations are identified by an IA value that differs significantly from zero.
(2) LD was estimated by the χ2 using the Arlequin program (Excoffier et al., Reference Excoffier, Laval and Schneider2005). With sexual reproduction, low LD values are expected for the vast majority of tested marker pairs. Moreover, pair-wise LDs should negatively correlate with physical distances between loci. To estimate the correlation between LD and physical distances for SSR marker pairs, LD was expressed as a negative logarithm of the P-value for the corresponding χ2 statistic. As with the IA-based test, a non-significant LD indicates sexuality in the tested populations.
3. Results
A slight tendency for decreasing LD with increasing distance between SSR loci was found in the EC I population (P<0·03) and the subpopulations of NFSEC I (P=0·11) and NFSEC II (P=0·25). This pattern, together with the relatively low level of LD, may point to clonal reproduction mixed with sex and recombination. In the EC III population with more marker pairs used, a significant correlation between LD and physical distances between the markers was obtained for the NFS subpopulation (Fig. 1). In the EC III population, particularly in its VB subpopulation, we can also see a tendency, although non-significant, for decreasing LD with growing physical distance between SSR loci. This tendency suggests that recombination may be present in this population. No significant correlation between LD and physical distance was found for the EC II populations, indicating the presence of clonal propagation in this population.
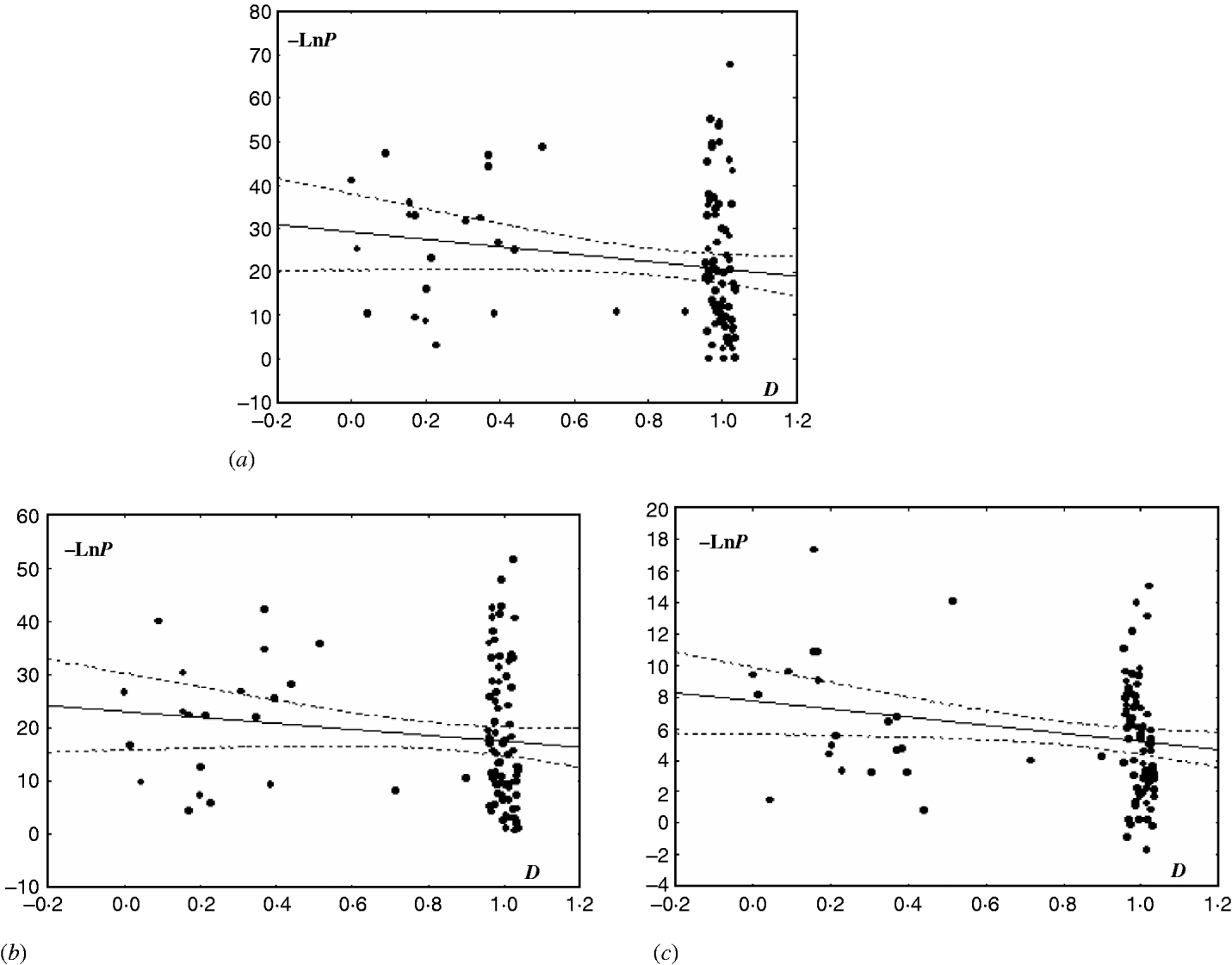
Fig. 1. Correlation between LD and physical distance (D) between pairs of tested loci in the populations and subpopulations of E. nidulans from EC III. Distance D was taken as equal to 1 for loci from non-homologous chromosomes (for obvious reasons, 1+ε was used instead of 1, with ε uniformly distributed in [−0·05, 0·05]) and equal to the proportion of distance between loci to chromosome length (in bp) for loci from the same chromosome. LD was scored as −Ln of P-value in the χ2-test. The graphs show the scored correlation between Y and X with 95% confidence (StatSoft 1996). (a) For EC III, R=−0·17, P=0·09, −LnP=29·24–8·49*D; (b) for EC III (VB), R=−0·14, P=0·17, −LnP=23·01–5·58*D; (c) EC III (NFS), R=−0·21, P=0·04, −LnP=7·78–2·58*D.
The results on LD (χ2-test) and IA for the E. nidulans populations are shown in Table 2. For the EC I and EC II populations, only 13% and 33% of the χ2 values from the pairwise comparisons, respectively, were significant. In contrast to EC I and EC II, for LD in the EC III population χ2 values were significant for 99% of marker pairs; a similar tendency was observed for IA. The obtained results point to the presence of sexuality in the EC I and EC II populations and predominant clonality of the EC III population.
Table 2. LD (χ2-test, below the diagonal) and IA (above the diagonal) for the populations of E. nidulans from EC I (a), EC II (b) and EC III (c)
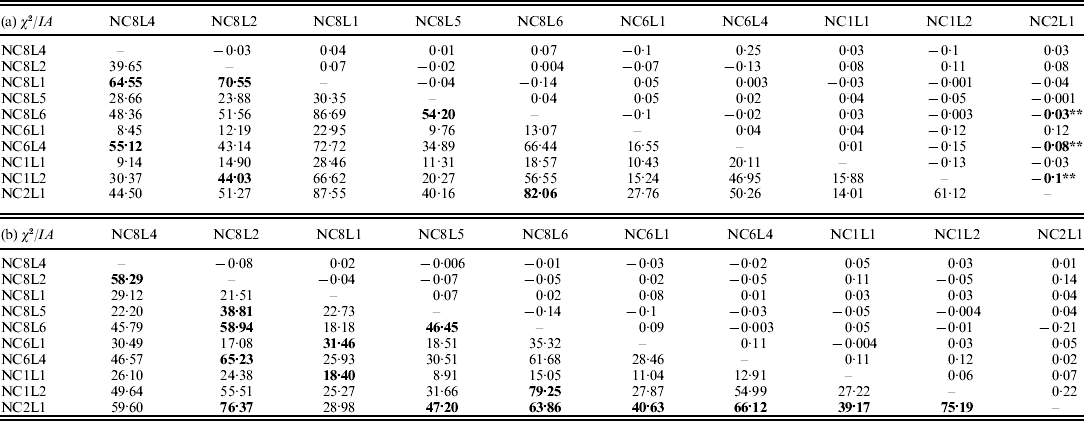
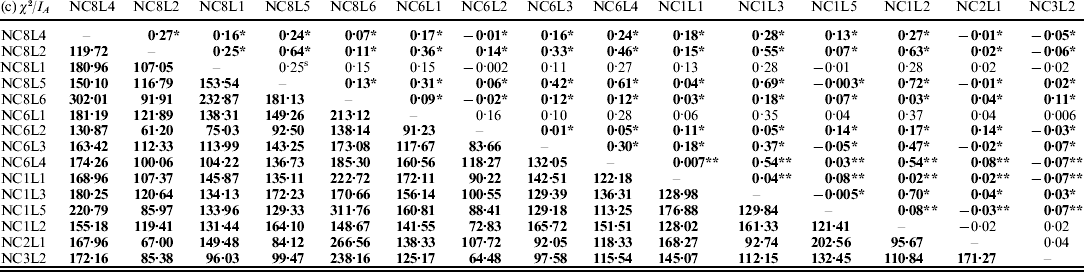
* P<0·05 and **P<0·005. Significant values of χ2 are bolded.
A significant positive correlation was obtained between the variance in the marker repeat number and physical distance from the markers to the centromere (R=0·314, P=0·009). Such a correlation can serve as additional evidence of the recombination presence in the tested populations. Similar to many other organisms, in E. nidulans the proximity to the centromere is known to greatly reduce the rate of recombination, a phenomenon referred to as the ‘centromeric effect’ (Korol et al., Reference Korol, Preygel and Preygel1994; Aleksenko et al., Reference Aleksenko, Nielsen and Clutterbuck2001; Espeso et al., Reference Espeso, Cobeno and Arst2005).
According to all estimated characteristics and additional tests (scoring of the correlation between inter-strain digital distances for each pair of loci in the populations and the bootstrap consensus analysis – data not shown), we consider the EC I and EC II populations to be predominantly sexual, in contrast to the EC III population, which showed a predominantly clonal structure.
4. Discussion
For multilocus marker systems, scoring IA and measuring LD by χ2-test has been traditionally used in examining whether a sexual or clonal mode of propagation prevails in natural, including fungal, populations (Maynard Smith et al., Reference Maynard Smith, Smith, O'Rourke and Spratt1993; Haubold et al., Reference Haubold, Travisano, Rainey and Hudson1998; Walser et al., Reference Walser, Gugerli, Holderegger, Kuonen and Scheidegger2004; Tuthill, Reference Tuthill2004; Stukenbrock & Rosendahl, Reference Stukenbrock and Rosendahl2005). The correlation between LD and physical distances was recently used for studying the reproductive structure of fungal populations (e.g. Tsai et al., Reference Tsai, Bensasson, Burt and Koufopanou2008) in spite of some opposite data showing that a negative correlation between polymorphism and DNA distance did not prove the presence of recombination and could probably be caused by different independent mutations (Hey, Reference Hey2000). In our study, both the IA and LD criteria as well as other methods (including correlation between genetic diversity indices and distance of SSR markers to the centromere) showed similar results. In the northern ECs, sexual propagation seems to contribute significantly to the E. nidulans' population structure, corroborating the findings on British populations of this fungus where recombination was shown to occur rather frequently (Geiser et al., Reference Geiser, Arnold and Timberlake1994). A different pattern was displayed by yeast Saccharomyces cerevisiae from the northern EC I, where the population proved to be heterogeneous for ploidy level, with diploids showing sexual structure while tri- and tetraploids were predominantly clonal (Katz Ezov et al., Reference Katz Ezov, Boger-Nadjar, Frenkel, Katsperovski, Kemeny, Nevo, Korol and Kashi2006).
All tests employed indicated different predominant types of reproduction in the northern and desert populations of E. nidulans. Predominant clonality was found in the EC III populations inhabiting the extreme desert environment. This finding seems to contradict the conjecture that the sexual stage prevails in stressful conditions (e.g. Elliot, Reference Elliot1994) and our previous results on the relationship between ecological stress and reproduction mode in soil microfungi (Grishkan et al., Reference Grishkan, Korol, Nevo and Wasser2003a). In that study, we showed a highly significant increase in the proportion of morphologically sexual species in mycobiota with an increasing salinity/aridity stress southwards in Israel. The question is whether this is really a contradiction. The presence of a morphologically expressed sexual stage in a homotallic fungus does not necessarily mean recombination occurs at the level of its population structure because of the ability of selfing. For example, Eupenicillium sp. with a known sexual stage in its life cycle was found to be predominantly clonal by molecular methods (Tuthill, Reference Tuthill2004). All of our desert isolates easily produced a selfing sexual stage under laboratory conditions. Presumably, ascospores may be produced in natural desert E. nidulans populations.
An environment-based explanation of the predominantly clonal character of the desert population of E. nidulans may be associated with the assumption that for relevant multilocus systems of a fungus, only several gene combinations (haplotypes) can exist under extremely stressful conditions (such as high solar and UV radiation, very low moisture and organic matter content, see Grishkan et al., Reference Grishkan, Beharav, Kirzhner and Nevo2007). Our results imply that in the most extreme, but stable, conditions asexual propagation might be preferential, corroborating several other studies (e.g. Murtagh et al., Reference Murtagh, Dyer and Crittenden2000). For E. nidulans grown under laboratory conditions, carbon deficiency, light exposure and high salinity were shown to preferentially stimulate asexual reproduction of the fungus, while less edaphically stressful conditions favoured sexual development (Kap-Hoon et al., Reference Kap-Hoon, Dong-Beom, Jong-Hak, Min-Su, Kyu-Yong, Won-Shin, Young-Soon, Heui-Baik and Dong-Min2003). Additionally, very low density of E. nidulans in the soil of the desert EC, which reduces the probability of finding a sexual partner, might favour predominant clonality via selfing. A similar tendency was found in marginal plant populations (e.g. Levin, Reference Levin1975; Nasrallah et al., Reference Nasrallah, Liu, Sherman-Broyles, Boggs and Nasrallah2004) and pioneer lichens (Murtagh et al., Reference Murtagh, Dyer and Crittenden2000), where selfing prevailed.
Many fungal populations seem to utilize mixed reproductive strategies (reviewed in Milgroom, Reference Milgroom1996) with different proportions of sexuality and clonality. Recently, some evidence of the sexuality in species with unknown sexual stages was reported, e.g., in A. fumigatus (Dyer & Paoletti, Reference Dyer and Paoletti2005), Aspergillus niger and Penicillium chrysogenum (Braumann et al., 2008). Our results cannot reject the hypothesis that increasing sexuality in fungi relates to increasing stress severity. Such a tendency may be a general rule, but becomes invalid at too extreme stresses (at the ‘edge of life’). Under such conditions, low population density (hence, the availability of a sexual partner) makes reproductive assurance a more important problem than the generation of recombination-dependent variation.
Another aspect of the problem is related to the interpretation of environmental severity. In addition to abiotic stress, one should consider the biotic components of the habitat. Competition stress (pressure of competitive fungal species) increased towards more mild ecological conditions in northern ECs (Grishkan et al., Reference Grishkan, Nevo, Wasser and Pavlicek2000, Reference Grishkan, Nevo, Wasser and Beharav2003b, Reference Grishkan, Beharav, Kirzhner and Nevo2007). Biotic stress is no less important to the evolution of sexuality than abiotic stress, as expressed in the Red Queen hypothesis of sex evolution (Hamilton et al., Reference Hamilton, Axelrod and Tanese1990; Korol et al., Reference Korol, Preygel and Preygel1994; Gandon & Otto, Reference Gandon and Otto2007). This can explain the observed tendency of increasing sexuality in E. nidulans towards increasing biotic stress in the NFS populations at the northern ECs.
Our results demonstrate the suitability of fungi for testing the effect of environmental stress on the reproduction mode when conducted in the context of natural populations inhabiting contrasting ecological conditions. Further studies are needed to discriminate between the two main explanations of the observed difference in reproductive strategies of E. nidulans in the inspected populations, i.e., reproductive assurance and biotic stress.
This work is in partial fulfilment of the requirements for the Ph.D. degree of E. Hosid. The study was supported in part by the Authority of Graduate Studies of the University of Haifa, Israeli Ministry of Absorption and the Ancell–Teicher Research Foundation for Genetics and Molecular Evolution.





