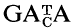 probes
probesPublished online by Cambridge University Press: 14 April 2009
The sex-reversal mutation, Sxr and a variant form, Sxr′ have been established on the inbred C57BL/6Mcl background by repeated backcrossing to form the CB and CB′ strains, respectively. DNAs of normal XY, XX Sxr and XX Sxr′ as well as XY Sxr and XY Sxr′ carrier male mice have been digested with the restriction enzymes Hae III and Hinf I and electrophoresed. The DNAs show many common but also differing hybridization bands with synthetic 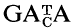 oligonucleotide probes. In XY Sxr (and XY Sxr′) carrier males, the hybridization patterns of normal XY and those of XX Sxr (and XX Sxr′) males are simply superimposed. Individual differing bands can be categorized by their differential hybridization behaviour to the (GATA)4, (GACA)4, (GATA)2 GACA (GATA)2 and (GATA)3 (GACA)2 probes. In general, the hybridization patterns are regularly inherited. In addition to the predominant pattern in each strain, one additional XX Sxr and one additional XX Sxr′ hybridization pattern was observed: the additional pattern in the CB strain was transmitted (via variant XY Sxr carriers) while the secondary XX Sxr′ pattern in the CB′ strain could only be observed once. Thus ‘DNA finger printing’ with
oligonucleotide probes. In XY Sxr (and XY Sxr′) carrier males, the hybridization patterns of normal XY and those of XX Sxr (and XX Sxr′) males are simply superimposed. Individual differing bands can be categorized by their differential hybridization behaviour to the (GATA)4, (GACA)4, (GATA)2 GACA (GATA)2 and (GATA)3 (GACA)2 probes. In general, the hybridization patterns are regularly inherited. In addition to the predominant pattern in each strain, one additional XX Sxr and one additional XX Sxr′ hybridization pattern was observed: the additional pattern in the CB strain was transmitted (via variant XY Sxr carriers) while the secondary XX Sxr′ pattern in the CB′ strain could only be observed once. Thus ‘DNA finger printing’ with 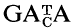 oligonucleotide probes can successfully be used to discriminate the DNAs of normal Y chromosomes, XX Sxr and XX Sxr′ variants as well as XY Sxr and XY Sxr′ carrier mice. Implications of the comparatively high unequal recombination rate involving the murine Y chromosome are discussed, as well as possible mechanisms.
oligonucleotide probes can successfully be used to discriminate the DNAs of normal Y chromosomes, XX Sxr and XX Sxr′ variants as well as XY Sxr and XY Sxr′ carrier mice. Implications of the comparatively high unequal recombination rate involving the murine Y chromosome are discussed, as well as possible mechanisms.