1. Introduction
The fragile X mental retardation 1 (FMR1) gene, is localized to Xq27·3, and contains a repetitive DNA segment, the (CGG)n trinucleotide. Mutation status depends on the number of CGG repeats, women who carry 58–200 repeats and those who carry more than 200 repeats are designated premutation and full mutation carriers of the Fragile X syndrome (MIM # 300624), respectively (Willemsen et al., Reference Willemsen, Levenga and Oostra2011). One of the phenotypes reportedly associated with having a long CGG repeat, is premature ovarian failure, or primary ovarian insufficiency (Wittenberger et al., Reference Wittenberger, Hagerman, Sherman, McConkie-Rosell, Welt, Rebar, Corrigan, Simpson and Nelson2007). Moreover, diminished ovarian reserve was also reportedly associated with a CGG repeat size that is not in the range of the “normal size repeat”, defined as 26–34 repeats (Gleicher et al., Reference Gleicher, Weghofer and Barad2010 a , 2010 b). Diminished ovarian reserve was also noted in BRCA1/BRCA2 mutation carriers (Oktay et al., Reference Oktay, Kim, Barad and Babayev2010). These phenotypic similarities in ovarian reserve, have led Weghofer and co-workers to analyze FMR1 CGG repeat length in BRCA1 and BRCA2 mutation carriers (Weghofer et al., Reference Weghofer, Tea, Barad, Kim, Singer, Wagner and Gleicher2012). They report that BRCA1/BRCA2 mutation carriers almost exclusively show low CGG repeats, with significant allele distribution differences between mutation carriers and noncarrier infertile controls. They conclude that this unique FMR1 CGG allele distribution in BRCA1/BRCA2 carriers underlies many of the noncancerous ovarian phenotypes reported in mutation carriers. In addition, based on their results, Weghofer and co-workers hypothesized that the unique FMR1 CGG repeat alleles may underlie the so called “BRCA paradox”, where BRCA alleles in the embryo exert an anti-proliferative effect (that may account for embryonic lethality in BRCA null embryos) whereas in adult tissues lack of BRCA protein expression is associated with a strong proliferative effect that eventually leads to tumor formation in the relevant tissues, e.g. breast and ovarian.
The present study was performed to validate and expand this previous report, targeting Jewish Israeli BRCA1/BRCA2 mutation carriers and a large Jewish control population group.
2. Materials and methods
(i) Study participants
Individuals counselled and genotyped for BRCA1/BRCA2 germline mutations or FMR1 CGG repeat size at the Danek Gertner Institute of Human Genetics at the Sheba Medical Center, Tel-Hashomer, Israel from 1 January 2008–31 December 2012 were eligible. The Oncogenetics Unit recruited women who were counselled and genotyped for being at high risk for developing breast/ovarian cancer as determined by standard criteria (Laitman et al., Reference Laitman, Simeonov, Herskovitz, Kushnir, Shimon-Paluch, Kaufman, Zidan and Friedman2012) and were subsequently found to harbour either a BRCA1 or BRCA2 mutation (see below). The control group was composed of women who were genotyped for their FMR1 CGG repeat length status in the context of prenatal genetic testing, offered in Israel. The study was approved by the ethics committee of the Sheba Medical Center and each participant gave an informed consent to participate in the approved study protocol.
(ii) Analysis of the predominant Jewish mutations in the BRCA1/BRCA2 genes
Mutational analyses for two of the three founder mutations (185delAG in BRCA1 and 6174delT in BRCA2) were carried out by restriction enzyme digest of PCR products, and analysis of the digested PCR products on agarose gels as previously described by us (Laitman et al., Reference Laitman, Simeonov, Herskovitz, Kushnir, Shimon-Paluch, Kaufman, Zidan and Friedman2012). For each of these mutations, a known mutation carrier was used as a positive control in each experiment and each mutation was confirmed by sequencing.
(iii) FMR1 CGG repeat analysis
FMR1 analyses were performed as previously reported (Gleicher et al., Reference Gleicher, Weghofer, Lee and Barad2011). Briefly, the FMR1 repeat region is amplified using a fluorescent primer, the PCR product will then be mixed with Liz 500 internal size standard (Applied Biosystems Inc., Foster City, CA, USA), and analyzed on the ABI Prism 3100 genotyper. The GeneScan raw data were analyzed using the GeneMapper software (version 3.1) to obtain the allele repeat in base pairs.
(iv) Statistical analyses
Proportions of FMR1 genotypes and sub-genotypes were compared between the two study groups using cross-tabulations and calculations of χ 2 and Cramer's V statistics. When comparing (CGG)n as a continuous function between the groups, nonparametric testing was used because (CGG)n in populations tends to be positively skewed. Mann – Whitney U tests were conducted to evaluate differences between the two groups in median change in (CGG)n of both alleles. All statistical calculations were performed utilizing SPSS, version 18 (Chicago, IL, USA).
3. Results
(i) Participant characteristics
(a) BRCA1/BRCA2 mutation carriers
Overall, there were 188 mutation carriers who participated in the study, 97 carried the BRCA1*185delAG mutation and 91 carried the BRCA2*6174delT mutation. There were 48 women who were diagnosed with breast cancer who harboured the 185delAG mutation and the mean age at diagnosis was 44·05 ± 12·1 years, and 45 breast cancer cases that carried the 6174delT mutation and the mean age at diagnosis was 44 ± 12·1 years. All other BRCA1/BRCA2 mutation carriers (n = 95) were cancer free (mean age at counselling and genotyping was 44 years for BRCA1 carriers and 44·2 years for BRCA2 mutation carriers).
(b) Controls
Overall 15 708 women underwent prenatal genetic testing to define the repeat length of the CGG trinucleotide repeat in the FMR1 gene in order to evaluate the need for prenatal diagnosis of embryonic Fragile X syndrome (Toledano-Alhadef et al., Reference Toledano-Alhadef, Basel-Vanagaite, Magal, Davidov, Ehrlich, Drasinover, Taub, Halpern, Ginott and Shohat2001; Berkenstadt et al., Reference Berkenstadt, Ries-Levavi, Cuckle, Peleg and Barkai2007). The mean age at counselling and genotyping was 26·7 ± 4·3 (19–37) years and 59% were of Ashkenazi origin. Notably, these women did not undergo BRCA1/BRCA2 mutational analysis.
(ii) CGG FMR1 repeat length genotyping
In the control, non-BRCA mutation carrier population, one FMR1 allele repeat number range was 8–600 repeats, with a median allele size of 30 repeats, and the second allele repeat number range was 15–600 repeats (median allele repeat size 30 repeats). Notably, this FMR1 CGG repeat length distribution was similar to that reported in other, ethnically diverse populations (Gleicher et al., Reference Gleicher, Weghofer, Lee and Barad2010 b ; Gleicher et al., Reference Gleicher, Weghofer, Lee and Barad2011).
Amongst BRCA mutation carriers, one FMR1 allele repeat number range was 14–40 repeats with a median allele of 29 repeats, and the second allele repeat size range was 23–92 repeats with the median allele repeat number of 30 repeats.
FMR1 genotypes and sub-genotypes distribution in the control population is consistent with previous reports in the Israeli population (Toledano-Alhadef et al., Reference Toledano-Alhadef, Basel-Vanagaite, Magal, Davidov, Ehrlich, Drasinover, Taub, Halpern, Ginott and Shohat2001; Berkenstadt et al., Reference Berkenstadt, Ries-Levavi, Cuckle, Peleg and Barkai2007). FMR1 genotypes and sub-genotypes distribution amongst BRCA carriers shows a distinct distribution compared with the control population (Table 1 and Fig. 1)
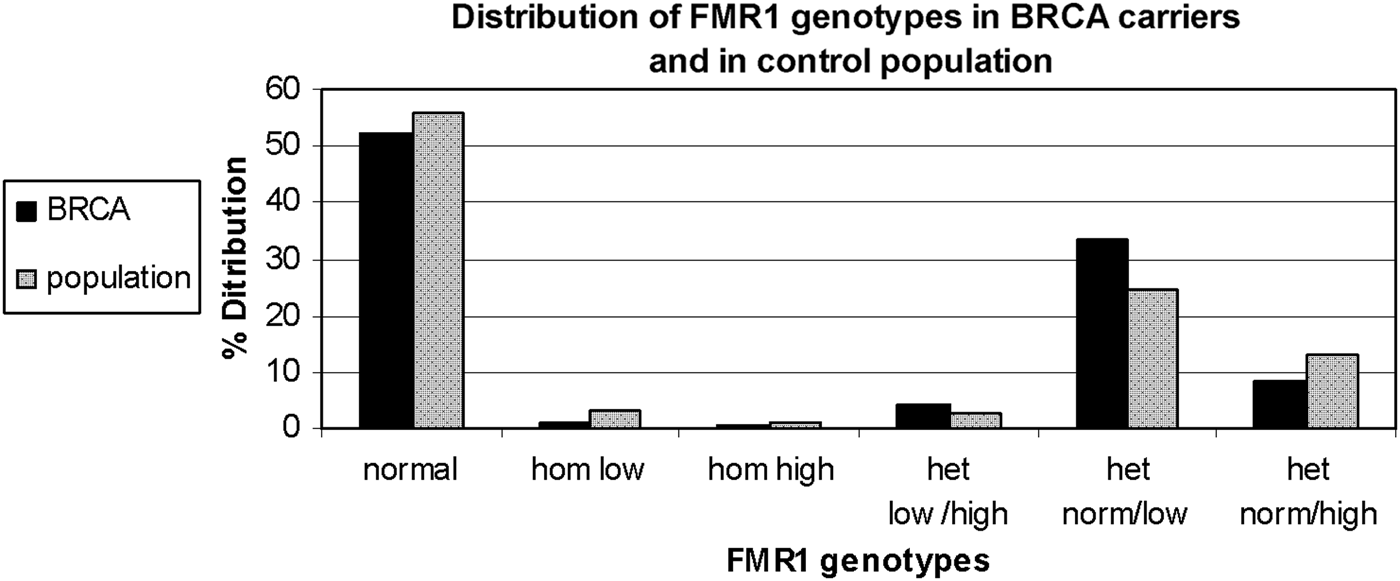
Fig. 1. FMR1 genotypes and sub-genotypes distribution in BRCA1/2 carriers (black bars) and the control population (grey bars).
Table 1. FMR1 genotypes and sub-genotypes distribution in BRCA1/2 carriers and the control population
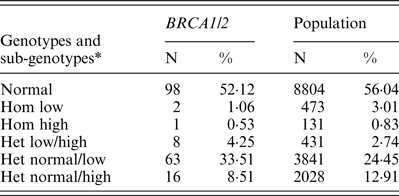
* Low alleles (n < 26); normal alleles (n = 26–34); high alleles (>35). het, heterozygous; hom, homozygous.
Comparison of genotypes distribution between the two study populations demonstrates a statistically significant difference (χ 2 = 13·612, d.f. = 5, p = 0·018)
When comparing allele distribution of the first FMR1 allele there is no difference in the distribution (Mann – Whitney p = 0·095). However, when comparing the allelic distribution of the second allele there is a marginally significant difference (Mann – Whitney p = 0·036) (Table 2). Notably, the rate of the normal/high alleles amongst heterozygote FMR1 CGG allele in the control group (12·9%) did not show any significant difference compared with BRCA mutation carriers (8·5%) (p = 0·073). Yet, the rate of the normal/low alleles amongst heterozygote FMR1 CGG allele in the control group (24·45%) showed a significant difference compared with BRCA mutation carriers (33·5%) (p = 0·004). There was no allele size distribution differences within BRCA carriers comparing cancer free (n = 95) and breast cancer affected women (n = 93) for the low allele (p = 0·19), for the high allele (p = 0·71) or for overall genotypes distribution (p = 0·43).
Table 2. FMR1 allelic distribution in BRCA1/2 carriers and the control population
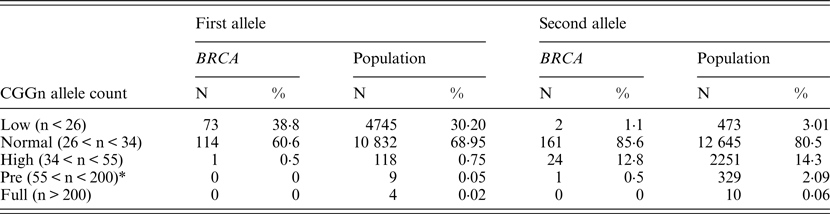
* Although the definition in Israel of permutation is currently 58 repeats, we elected to use the 55 repeats figure to facilitate comparison with the other studies.
4. Discussion
In the present study, the FMR1 CGG trinucleotide repeat length distribution among Jewish Israeli BRCA1/BRCA2 mutation carriers was different from that exhibited by the general Israeli, mostly Ashkenazi, population. These FMR1 CGG allele distribution differences results are in line with those of Weghofer et al. (Reference Weghofer, Tea, Barad, Kim, Singer, Wagner and Gleicher2012) who previously reported a unique FMR1 CGG allele distribution in BRCA1/BRCA2 mutation carriers undergoing fertility treatment compared with noncarriers recruited from an infertility clinic. Yet, unlike the almost complete absence of long CGG repeat sizes (34 repeats and above) in the study by Weghofer and co-workers (2012), in the present study 8·5% of BRCA1/BRCA2 carriers had at least one long CGG allele, a rate that was not statistically different to that of the control population (12·9%). The basic premise of the Weghofer et al. (Reference Weghofer, Tea, Barad, Kim, Singer, Wagner and Gleicher2012) study was that the long CGG allele in the presence of a BRCA mutation may be embryonic lethal, and that rescue may be conferred by the existence of short (<26 repeats) alleles; however, our results do not support this notion. In line with our previous data, smaller studies published from Israel (Dagan et al., Reference Dagan, Cohen, Mory, Adir, Borochowitz, Raanani, Kurolap, Melikhan-Revzin, Meirow and Gershoni-Baruch2014), Italy (Ricci et al., Reference Ricci, Pennese, Gismondi, Perfumo, Grasso, Gennaro, Bruzzi and Varesco2014) and Holland (Brandão et al., Reference Brandão, van Roozendaal, Tserpelis and Blok2013) also failed to show the lack of long CGG FMR1 alleles in BRCA mutation carriers. Another recent study that focused on ovarian cancer cases (n = 87) showed that ovarian cancer cases who are BRCA pathogenic mutation carriers (n = 15) exhibit a normal-range prevalence of FMR1 CGG alleles, whereas ovarian cancer patients who are BRCA wild-type, display a trend (not reaching statistical significance) towards higher prevalence of low CGG repeats of the FMR1 gene (Gleicher et al, Reference Gleicher, McAlpine, Gilks, Kushnir, Lee, Wu, Lazzaroni-Tealdi and Barad2014). Moreover, the data presented herein and the above mentioned previous reports (summarized in Table 3) also question the putative role attributed by Weghofer and co-workers (2012) to FMR1 alleles in mediating premature ovarian failure in BRCA mutation carriers.
Table 3. Summary of the studies focusing on CGG FMR1 alleles and BRCA mutation carriers
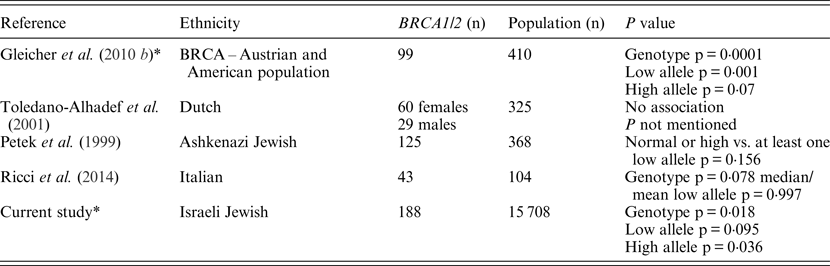
* Statistical analyses were performed using the same definitions and analysis tools.
Several reasons may underlie the discrepancy in the results on the rates of the longer CGG alleles between the original Weghofer and co-workers (2012) study and the subsequent studies: the ethnicity of the genotyped groups in both Israeli studies was different, with mostly Ashkenazi Jewish women genotyped in these studies compared with mostly Caucasian European and American women in the original study. These ethnic differences are reflected in the location and type of mutations in both BRCA1 and BRCA2 genes, and given the genotype – phenotype correlations reported in BRCA1/BRCA2 mutation carriers, the Weghofer and co-workers (2012) study results may be in part attributed to these genetic and ethnic differences. This is not a valid argument to account for the lack of replication in the Italian and Dutch studies. Another factor that may have led to the discordant results is the mode of recruitment and ascertainment of BRCA mutation carriers: a cancer and prenatal genetics clinic in the present study and the study by Dagan and co-workers (2014), a familial breast cancer clinic in the study by Ricci et al. (2014) and an infertility clinic in the Weghofer et al. (Reference Weghofer, Tea, Barad, Kim, Singer, Wagner and Gleicher2012) study. However, this latter explanation is unlikely to account for the inconsistent results, as previous studies reported a similar FMR1 CGG repeat length in both fertile and infertile women of diverse ethnicities (Toledano-Alhadef et al., Reference Toledano-Alhadef, Basel-Vanagaite, Magal, Davidov, Ehrlich, Drasinover, Taub, Halpern, Ginott and Shohat2001; Berkenstadt et al., Reference Berkenstadt, Ries-Levavi, Cuckle, Peleg and Barkai2007; Gleicher et al., Reference Gleicher, Weghofer, Lee and Barad2010 b ; Gleicher et al., Reference Gleicher, Weghofer, Lee and Barad2011).
Unique ovarian-reproductive phenotypes have previously been reported in BRCA1 and BRCA2 mutation carriers, in addition to ovarian cancer the following have been observed: an increased risk for recurrent miscarriages (Gal et al., Reference Gal, Sadetzki, Gershoni-Baruch, Oberman, Carp, Papa, Diestelman-Menachem, Eisenberg-Barzilai and Friedman2004), a skewed ratio of male to female offspring (de la Hoya et al., Reference de la Hoya, Fernández, Tosar, Godino, Sánchez de Abajo, Vidart, Pérez-Segura, Díaz-Rubio and Caldés2003). The mechanisms for these noncancerous phenotypes and the pathways that underlie them are presently unclear. It is possible that the unique FMR1 CGG allele size, as reflected by the different allelic distribution between cases and controls in our study, plays a role in these additional phenotypes, but currently this possibility remains speculative at best.
The data presented also make it unlikely that the FMR1 CGG repeat length size plays a significant role in affecting breast cancer penetrance in BRCA1 or BRCA2 mutation carriers, as the allele distribution was similar in breast cancer affected women and in ethnically matched cancer-free mutation carriers. This is further emphasized by the results of Gleicher and co-workers (2014) in BRCA carriers diagnosed with ovarian cancer, displaying a normal range of FMR1 CGG alleles. It is noteworthy that the present study and all previous studies are underpowered to detect any subtle effect of the FMR1 CGG genotype on BRCA1/BRCA2 penetrance.
In conclusion, the CGG trinucleotide repeat in the FMR1 gene in Jewish Israeli women, predominantly of Ashkenazi origin, shows a distinct distribution among BRCA1/BRCA2 mutation carriers compared with the general population, but these differences are unlikely to account for the common phenotype of premature ovarian failure.
This study was sponsored in part by a grant from the Israeli Cancer Association to Eitan Friedman in the context of the Israeli inherited breast cancer consortium.
Declaration of interest
None.






