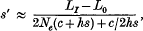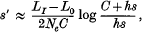Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Kimura, Motoo
1971.
Theoretical foundation of population genetics at the molecular level.
Theoretical Population Biology,
Vol. 2,
Issue. 2,
p.
174.
Charlesworth, Brian
and
Charlesworth, Deborah
1973.
Selection of new inversions in multi-locus genetic systems.
Genetical Research,
Vol. 21,
Issue. 2,
p.
167.
Ohta, Tomoko
1973.
Effect of linkage on behavior of mutant genes in finite populations.
Theoretical Population Biology,
Vol. 4,
Issue. 2,
p.
145.
Lichter, Robert
1973.
Fortschritte der Botanik.
p.
233.
Ohta, Tomoko
and
Cockerham, C. Clark
1974.
Detrimental genes with partial selfing and effects on a neutral locus.
Genetical Research,
Vol. 23,
Issue. 2,
p.
191.
Kirby, G.C.
1975.
Heterozygote frequencies in small subpopulations.
Theoretical Population Biology,
Vol. 8,
Issue. 1,
p.
31.
Baptist, R.
1977.
The combined effect of pseudo‐overdominance and migration on genetic variability in a small simulated population.
Zeitschrift für Tierzüchtung und Züchtungsbiologie,
Vol. 94,
Issue. 1-4,
p.
302.
Barker, J.S.F.
1979.
Inter-locus interactions: A review of experimental evidence.
Theoretical Population Biology,
Vol. 16,
Issue. 3,
p.
323.
Malpica, J. M.
and
Briscoe, D. A.
1982.
Multilocus nonrandom associations inDrosophila melanogaster.
Genetical Research,
Vol. 39,
Issue. 1,
p.
41.
Zouros, E.
and
Foltz, D. W.
1983.
Minimal selection requirements for the correlation between heterozygosity and growth, and for the deficiency of heterozygotes, in oyster population.
Developmental Genetics,
Vol. 4,
Issue. 4,
p.
393.
Zouros, E.
Singh, Shiva M.
Foltz, David W.
and
Mallet, André L.
1983.
Post-settlement viability in the American oyster (Crassostrea virginica): an overdominant phenotype.
Genetical Research,
Vol. 41,
Issue. 3,
p.
259.
Butlin, R K
and
Day, T H
1985.
Genie and karyotypic selection on an inversion polymorphism in the seaweed fly, Coelopa frigida.
Heredity,
Vol. 54,
Issue. 2,
p.
267.
Santos, M.
1986.
The role of genic selection in the establishment of inversion polymorphism in Drosophila subobscura.
Genetica,
Vol. 69,
Issue. 1,
p.
35.
Danzmann, Roy G.
Ferguson, Moira M.
Allendorf, Fred W.
and
Knudsen, Kathy L.
1986.
HETEROZYGOSITY AND DEVELOPMENTAL RATE IN A STRAIN OF RAINBOW TROUT (
SALMO GAIRDNERI
)
.
Evolution,
Vol. 40,
Issue. 1,
p.
86.
Chakraborty, Ranajit
1987.
Biochemical heterozygosity and phenotypic variability of polygenic traits.
Heredity,
Vol. 59,
Issue. 1,
p.
19.
Zouros, E.
Romero-Dorey, M.
and
Mallet, A. L.
1988.
HETEROZYGOSITY AND GROWTH IN MARINE BIVALVES: FURTHER DATA AND POSSIBLE EXPLANATIONS.
Evolution,
Vol. 42,
Issue. 6,
p.
1332.
Dillon, Robert T.
and
Manzi, John J.
1988.
Enzyme heterozygosity and growth rate in nursery populations of Mercenaria mercenaria (L.).
Journal of Experimental Marine Biology and Ecology,
Vol. 116,
Issue. 1,
p.
79.
Pogson, Grant H.
1989.
Biochemical characterization of genotypes at the phosphoglucomutase-2 locus in the pacific oyster,Crassostrea gigas.
Biochemical Genetics,
Vol. 27,
Issue. 9-10,
p.
571.
Pogson, Grant H.
1989.
Biochemical characterization of genotypes at the phosphoglucomutase-2 locus in the pacific oyster,Crassostrea gigas.
Biochemical Genetics,
Vol. 27,
Issue. 9-10,
p.
571.
Volckaert, F.
and
Zouros, E.
1989.
Allozyme and physiological variation in the scallop Placopecten magellanicus and a general model for the effects of heterozygosity on fitness in marine molluscs.
Marine Biology,
Vol. 103,
Issue. 1,
p.
51.