1. Introduction
Tropical savannas have been heavily impacted by anthropogenic land-cover conversion for agriculture. Agriculture now occupies nearly 1 million km2 of the Brazilian “Cerrado” savanna, or ~ 50% of the biome’s original extent (Lapola et al., Reference Lapola, Martinelli, Peres, Ometto, Ferreira, Nobre, Aguiar, Bustamante, Cardoso, Costa, Joly, Leite, Moutinho, Sampaio, Strassburg and Vieira2014). However, after short periods of use many of the marginal agricultural areas are abandoned, particularly in smallholdings (<100 ha), which represent approximately 25% of agricultural land-cover (Lapola et al., Reference Lapola, Martinelli, Peres, Ometto, Ferreira, Nobre, Aguiar, Bustamante, Cardoso, Costa, Joly, Leite, Moutinho, Sampaio, Strassburg and Vieira2014).
Regenerative agroforestry has been shown to recover productive biodiverse ecosystems from abandoned degraded lands in tropical Brazil (Vieira et al., Reference Vieira, Holl and Peneireiro2009; Yamada & Gholz, Reference Yamada and Gholz2002). Although attention has focused on Amazonian (Browder et al., Reference Browder, Wynne and Pedlowski2005; Yamada & Gholz, Reference Yamada and Gholz2002) and Atlantic Forest (Ferreira et al., Reference Ferreira, Peres, Dodonov and Cassano2020; Santos et al., Reference Santos, Crouzeilles and Sansevero2019) regions, smallholders in savanna regions are also likely to benefit from regenerative agroforestry (Lambers et al., Reference Lambers, de Britto Costa, Oliveira and Silveira2020; Miccolis et al., Reference Miccolis, Peneireiro, Vieira, Marques and Hoffmann2017). Wildfires are the main determinants of vegetation composition in savannas (Chidumayo, Reference Chidumayo2013), yet empirical evidence documenting how fire influences regenerative agroforestry in Brazilian savanna smallholdings remains scarce.
Fire (Moura et al., Reference Moura, Scariot, Schmidt, Beatty and Russell-Smith2019) and seed predation (Ferreira et al., Reference Ferreira, Bruna and Vasconcelos2011) are expected to strongly influence regenerative potential of degraded Brazilian savannas. The use of fire remains controversial for the management and restoration of Brazilian savanna areas (Carmenta et al., Reference Carmenta, Coudel and Steward2019; Eloy et al., Reference Eloy, Bilbao, Mistry and Schmidt2019). Trees may be killed by fire and if fire regimes do not allow seed banks to recover before repeated ignition events it may even cause local extinctions (Auld et al., Reference Auld, Denham and Turner2007). Alternatively fire can serve as a trigger for germination, release nutrients into the soil and prepare soil surface to receive new seeds. Seed mortality is high in tropical regions due to predation, desiccation and rot (Cole, Reference Cole2009). Thus, seed predation plays a key role in structuring plant populations and communities with post-dispersal seed predation causing extensive seed loss (Curran & Webb, Reference Curran and Webb2000; Ferreira et al., Reference Ferreira, Bruna and Vasconcelos2011; Hulme, Reference Hulme1998).
2. Objectives
Our objective was to compare seed infestation and removal in burned and unburned savanna areas. We hypothesize that burned savanna areas will have lower seed removal/infestation compared with unburned areas. To test this hypothesis we experimentally compared seed removal and seed infestation rates of seeds from two non-legume species [popcorn kernels (Zea mays L. – Poaceae) and açaí fruits (Euterpe oleracea Mart. – Arecaceae, Supplemental Material S1] in one burned and two unburned savanna areas.
3. Methods
The study was conducted at the Campo Experimental do Cerrado (CEC) of Embrapa-Amapá (0°2′ 5”N, 51°2′ 2”W, Figure 1). A month before the study wildfire passed across part of the CEC, providing an ideal natural experiment to test seed removal in burned and unburned savanna habitats. We set our experiment in three habitat types (Figure 1, Supplemental Material S2): burned savanna (BS) with the most recent wildfire recorded one month before the experiment, unburned savanna (US) with over 20 years without any record of wildfire, and unburned riparian savanna forest (RF) with no record of wildfire.
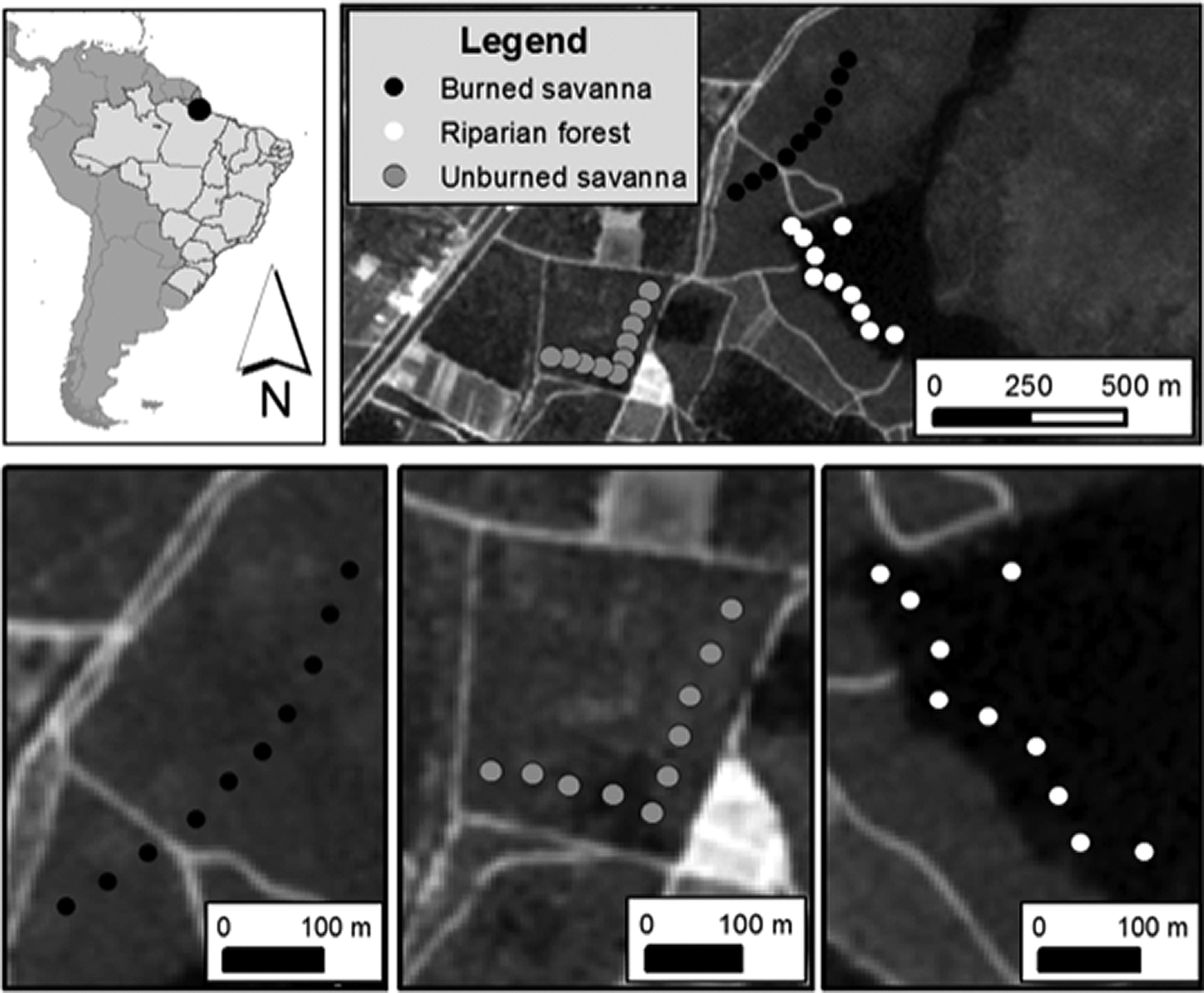
Figure 1. Location of the study region in the Campo Experimental do Cerrado (CEC) of Embrapa-Amapá, Amapá state, Brazil, showing the 30 seed removal units monitored (10 black, 10 white and 10 grey circles representing burned savanna, riparian savanna forest, and unburned savanna, respectively).
In each habitat type (BS, US, and RF) we established 10 experimental units (five per seed type), 50 m apart from each other (Kaspari, Reference Kaspari1993). We alternated experimental units between palm fruits and popcorn kernels. In order to identify the seed removers we used three selective access treatments (Supplemental Material S3): (1) open access, with all seeds or fruits placed on the soil surface so that any bird, mammal or invertebrate could access; (2) vertebrate access, when ants and other invertebrates were excluded by a 2–3 cm circular moat around the seeds/fruits; and (3) invertebrate access, where vertebrates were excluded by covering the seeds/fruits with a protective wire tower (5 mm mesh, 15–20 cm high) (Christianini & Galetti, Reference Christianini and Galetti2007; Ferreira et al., Reference Ferreira, Bruna and Vasconcelos2011; Norris & Michalski, Reference Norris and Michalski2010; Tasker et al., Reference Tasker, Denham, Taylor and Strevens2011). In each experimental unit, the three treatments each contained 10 seeds or fruits separated by 1 m. To avoid confounding edge effects experimental units were placed along transects that were established at a fixed distance of 50 m from any nearest habitat edge. We monitored experiments for four consecutive days, providing a total of 79 observation hours at each habitat type simultaneously, between 13 and 16 November 2015 (see Supplemental Material S2 and S3 for further details). Although previous studies in Brazilian savannas demonstrate that most seed/fruit removal occurred within 24 h (Alexander et al., Reference Alexander, Antônio and Oliveira2007; Ferreira et al., Reference Ferreira, Bruna and Vasconcelos2011) we allowed seeds to remain exposed for four days to provide a more complete assessment of seed removal and infestation rates (Ferreira et al., Reference Ferreira, Bruna and Vasconcelos2011).
The fate of kernels/fruits was classified as removed, infested (when invertebrates entered internally e.g. entered beyond the exocarp) or intact. To identify if kernel/fruit removal and infestation varied among habitats, seed type and treatments we used Generalized Linear Models (GLMs, binomial error distribution). Variograms (Figure 2) were used to test for spatial autocorrelation in model residuals (Cressie, Reference Cressie1993; Legendre, Reference Legendre1993). All analyses were conducted in R (R Development Core Team, 2019), with functions available in geoR (Ribeiro Jr & Diggle, Reference Ribeiro and Diggle2001) and tidyverse (Wickham et al., Reference Wickham, Averick, Bryan, Chang, McGowan, François, Grolemund, Hayes, Henry, Hester, Kuhn, Pedersen, Miller, Bache, Müller, Ooms, Robinson, Seidel, Spinu and Yutani2019) packages.
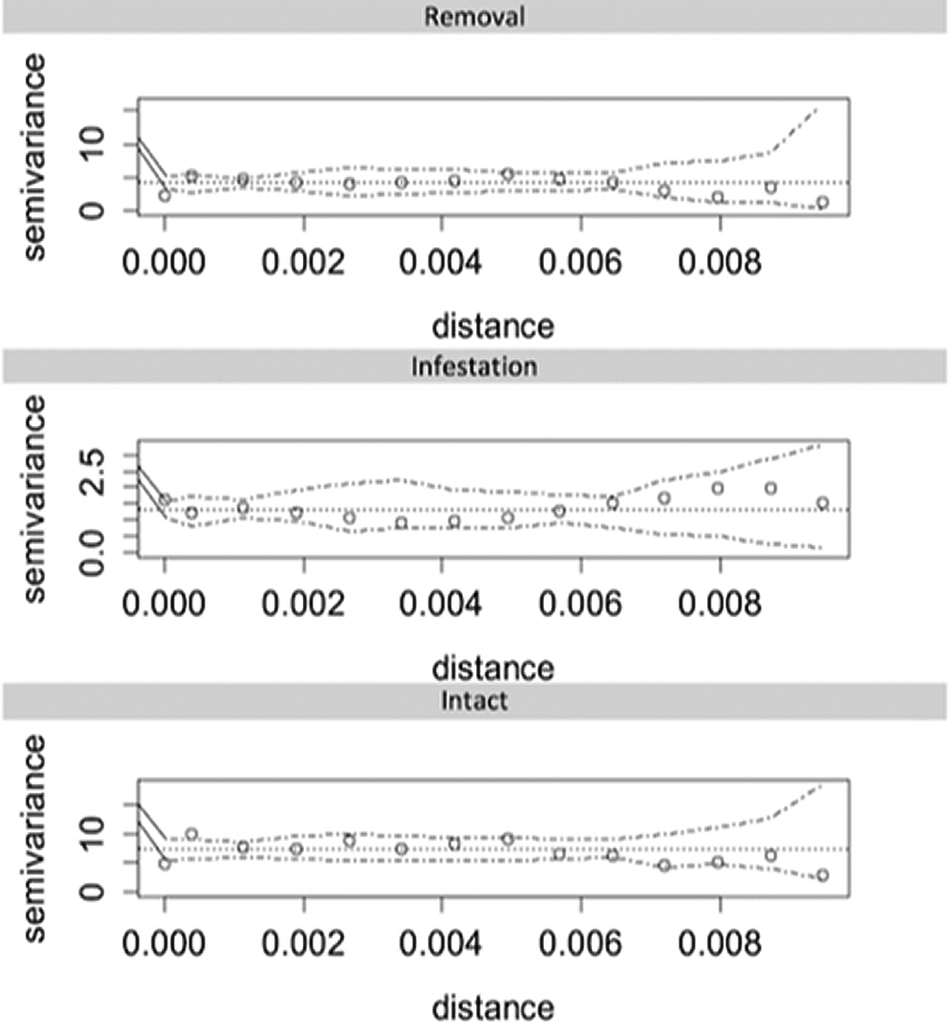
Figure 2. Sample semi-variograms and simulation envelopes under random permutation of GLM residuals. Distance calculated from geographic coordinates (decimal degrees, 0.001 ≈ 100 m).
4. Results
The type of habitat, treatment and seed had significant effects on the probability of removal and infestation (Figure 3, Table 1). More popcorn kernels remained intact compared with açaí fruits (Figure 3, Table 1). Although popcorn experienced increased removal there was less infestation compared with açaí (Figure 3, Table 1). The number of fruits and kernels removed (P < 0.0001) and infested (P < 0.0001) varied significantly among habitat types (Figure 3, Table 1). The greatest removal rates (25.5%) were recorded in the riparian savanna forest, followed by the unburned and burned savannas, with 5.3% and 1.3% removal, respectively (Figure 3). The variograms used to test for spatial autocorrelation in model residuals (Figure 2) did not show spatial correlation for any treatment. Indeed, variograms showed a lack of pattern and/or trend, demonstrating that the unexplained variation in our GLMs was also not explained by spatial autocorrelation.
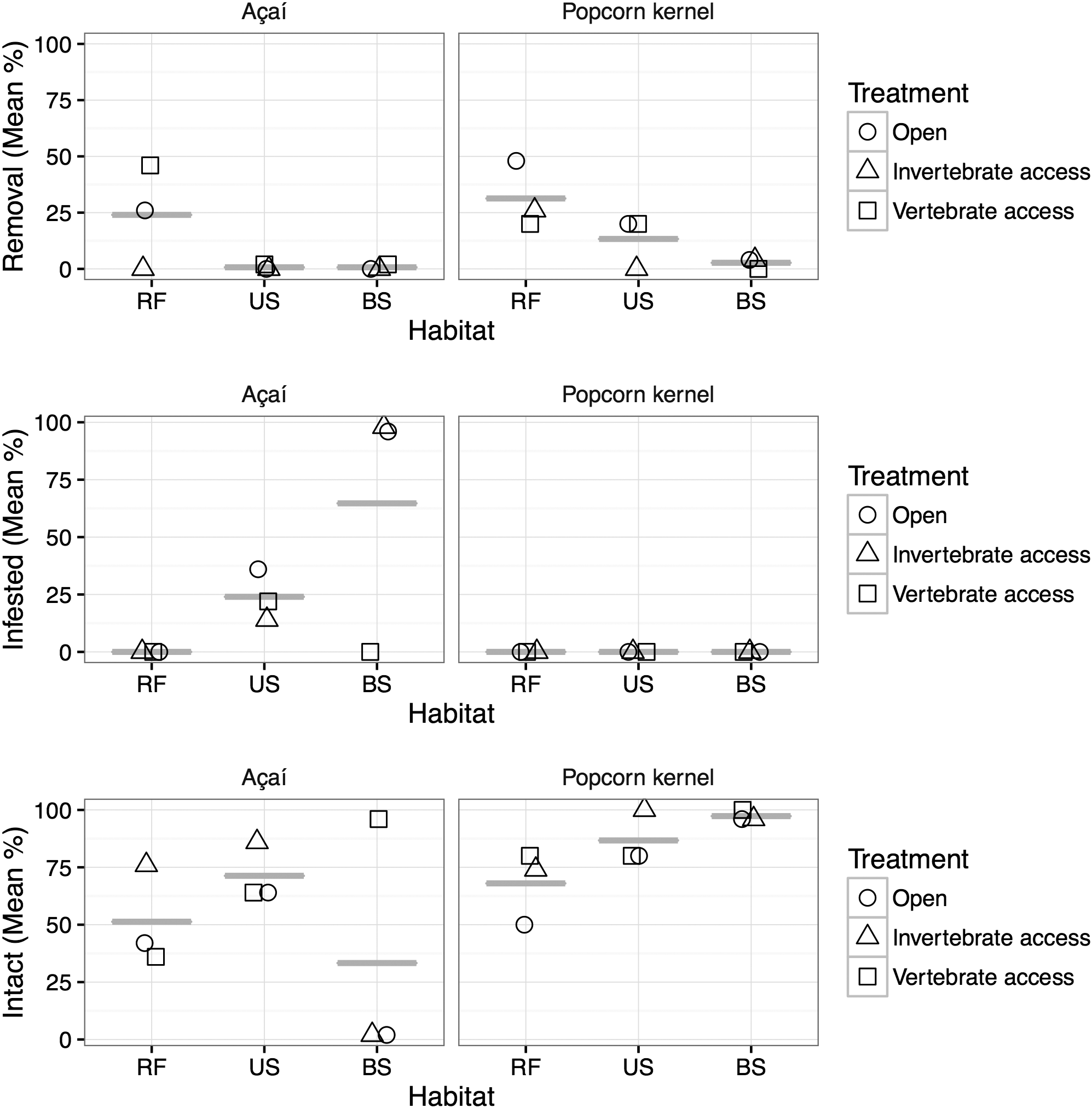
Figure 3. Short-term fate of açaí fruit and popcorn kernels in an Amazonian savanna. Comparison of percentage removed, infested and number of intact açaí fruits and popcorn kernels. Experiments were conducted in three habitats (RF – Riparian Savanna Forest, US – Unburned savanna, and BS – Burned savanna) in November 2015. In each habitat the fruits and kernels were placed in three treatments (Open, Invertebrate access, and Vertebrate access).
Table 1. Generalized Linear model results. Three binomial responses were used to examine short-term seed fate in an Amazonian savanna: i) Removal, ii) Infestation and iii) Number of intact seeds.
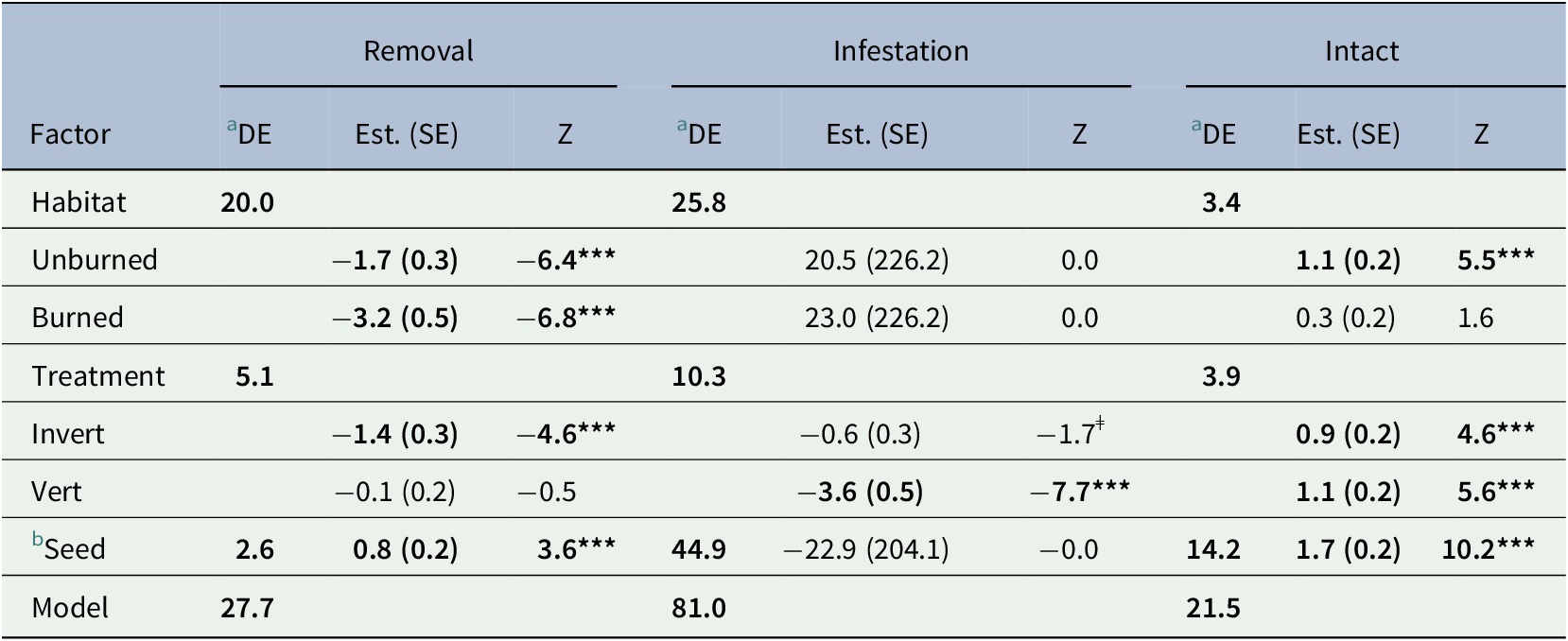
Signif. codes: ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘ǂ’ 0.1
a Percentage of model deviance explained.
b Popcorn compared to açaí.
Bold text indicates a statistically significant difference with a p < 0.05.
Neither fruits nor kernels were removed from burned areas, however 100% infestation occurred when invertebrates had access to açaí fruits in burned areas (Figure 3). The proportion of infested seeds/fruits was higher on average in the burned savanna (24.3%), followed by unburned savanna (9.0%), while the riparian savanna forest had no infested seeds/fruits.
5. Discussions
We found that vertebrate access increased removal of açaí fruits in the riparian savanna area. A preference for habitats with dense vegetation cover, in order to avoid predation while searching for food (Souza-Silva et al., Reference Souza-Silva, Machado, Silva and Espirito-Santo2015) could explain the higher vertebrate removal rates in the riparian savanna forest compared to more exposed burned and unburned savannas.
Our findings agree with previous studies that show how both insects and fire influence the structure and composition of savanna vegetation (Alexander et al., Reference Alexander, Antônio and Oliveira2007; Costa et al., Reference Costa, Vasconcelos and Bruna2017; Maravalhas & Vasconcelos, Reference Maravalhas and Vasconcelos2014; Oliveira & Freitas, Reference Oliveira and Freitas2004). The increased açaí infestation rates in the burned and unburned savanna habitats were related with the occurrence of termites, which were not observed in unburned riparian savanna forest. After only four days we demonstrated that 100% of invertebrate infestation occurred when invertebrates had access to açaí fruits in burned areas, which clearly demonstrates the differences in invertebrate infestation between burned and unburned areas.
Although previous studies have largely focused on the role of ants (Alexander et al., Reference Alexander, Antônio and Oliveira2007; Costa et al., Reference Costa, Vasconcelos and Bruna2017; Maravalhas & Vasconcelos, Reference Maravalhas and Vasconcelos2014; Oliveira & Freitas, Reference Oliveira and Freitas2004) our findings suggest that additional studies are needed to establish how termites influence recruitment and regeneration patterns in Brazilian savanna areas. Through diverse relationships with microorganisms termites may exploit living and/or dead plant tissues (Logan et al., Reference Logan, Cowie and Wood1990) and termite predation can affect seed viability (Nwosu & Akor, Reference Nwosu and Akor2018). The high infestation of seeds/fruits in burned savanna areas agrees with findings from South Africa (Davies et al., Reference Davies, Eggleton, van Rensburg and Parr2012) and Australia (Avitabile et al., Reference Avitabile, Nimmo, Bennett and Clarke2015) that show how termites are resistant to savanna fires. Additionally the rapid infestation rates in both burned and unburned habitats is likely to generate challenges for conservationists and managers to restore degraded areas in tropical savanna regions.
Our results should be interpreted carefully as we conducted our experiment for a short period and without more replicates within the different habitats. Although previous studies show that most removal occurs within 24 hours (Alexander et al., Reference Alexander, Antônio and Oliveira2007; Ferreira et al., Reference Ferreira, Bruna and Vasconcelos2011), it is possible that over time the removal and infestation levels may continue to increase in the different habitats. Considering that we did not establish if infestation affected seed viability, additional longer term (multi-year) experiments focusing on germination and recruitment with more species are needed to establish the viability of different agroforestry options in the region. As such our findings support previous studies (Lambers et al., Reference Lambers, de Britto Costa, Oliveira and Silveira2020; Miccolis et al., Reference Miccolis, Peneireiro, Vieira, Marques and Hoffmann2017) that show how the development of effective restoration and/or agroforestry will depend on detailed experimental evidence to enable development of the most cost and time effective actions in Brazilian savannas.
6. Conclusions
In this study, the type of habitat, treatment and seed had significant effects on the probability of seed removal and infestation. Overall, more popcorn kernels remained intact compared with açaí fruits. Yet, burned savanna areas showed 100% invertebrate seed infestation rates. These findings suggest that preparation burning in isolation may not be effective for regenerative agroforestry and that the management and restoration potential in burned Amazon savannas could be severely limited by invertebrate infestation.
Acknowledgements
We are deeply indebted for the Brazilian Agricultural Research Corporation (EMBRAPA) for providing research permit and logistical support. The Federal University of Amapá also provided logistical support. This article resulted from the PPGBIO field ecology course in 2015. This study is dedicated to the memory of Dr. Silas Mochiutti, whose kindness and curiosity inspired us all.
Author Contributions
DN, FM and SM conceived and designed the study. VJUR, TPS, JAS, KSC, IAPS, AFS, DSSV, SM and FM conducted data gathering. DN performed statistical analyses. VJUR, TPS, JAS, KSC, IAPS, AFS, DSSV, SM, DN and FM wrote the original draft. All authors revised the text.
Financial Support: Statement
We thank the Postgraduate Program in Tropical Biodiversity (PPGBIO) of the Federal University of Amapá for providing funding.
Conflicts of Interest
All authors declare none.
Data Availability Statement
All materials needed to replicate the findings of the article are available as Supplementary Materials.
Supplementary Materials
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/exp.2021.2.







Comments
Comments to the Author: The topic of the research, the effect of preparation burning on short-term seed survival in Amazonian savannas, is very interesting, with an important practical aspect. In general, the manuscript was well written, easy to follow and I found the statistical analyses to be appropriate. However, I have some comments and small suggestions for the authors.
Comments for the authors
In the title, the authors state that preparing burning doesn´t improve seed survival. This looks true when focusing on the great increase of infestation rate observed for palm seeds in the burned savanna. In contrast, the seed remotion rate for popcorn was the smallest in the burned savanna, reaching also the highest rate for intact seeds of popcorn. For me, this might indicate a species-specific effect, with a decrease of attacks on less attractive seeds (i.e., they without pulp, arils, or other attractive substances, as kernels), while it´d be observed an increase of attacks on fruits and seeds with pulp or arils, as fruits of Açaí). So, it´d be good for some species and worse for others. Could this hypothesis be considered?
This brings me to another question. Could the infestation by invertebrates (in this case, mainly termites, line 140) reduce the short-term survival of palm seeds? The cited references confirm the importance of invertebrates, however, mainly ants, on seed bank diversity, with potential consequences for the structure and composition of savanna vegetation. I confess that I have doubts whether termites would consume the seeds, behind to immediately explore only their fruit pulp. In the first situation, a negative impact on seed survival would be expected, but maybe not in the second case. Were the infested seeds consumed, at least in part, by insects? I think this important and suggest to the authors include some references that confirm termites, and consequently the referred insect infestation, as a potential seed predator and their presence with potential impact on short-term survival of seeds.
In the Abstract, the authors omitted the third habitat (riparian forest). However, the major difference in seed remotion occurred just between forest and savanna habitats for both palm and popcorn, which was likely the great responsible for the significant difference observed among habitats. So, I think that is correct to include the forest habitat in the result presentation of the abstract, with the appropriate adjustments.
Minor reviews
Line 33: Without cite the riparian forest in the abstract as a study habitat, it was strange to attest that “savanna” had the highest overall seed infestation rate. Higher than who?
Line 49: Remove the name initials in Ferreira et al, 2011 and 2020 across the text.
Line 70: Clarify in methods was considered insect infestation. Was the presence of insects on and/or in fruits and seeds?
Line 83: Throughout the text, the authors alternated the use of two terms: “riparian forest” or “riparian savanna forest”. What would be a riparian savanna forest? Isn´t possible to include a short description of vegetation found in study habitats. It´d be useful for readers unfamiliar with the Brazilian ecosystems.
Line 126: Replace ‘number’ with ‘rate’.
Line 136: Include the word ‘may’ in ‘how both insects and fire may influence the structure… I think this important to appear less emphatic. The same could be done in other parts of the text, as in the title: “… may not improve short-term …”, but it´s just a suggestion.