Introduction
Children with complex congenital heart disease (CHD) are at increased risk for neurodevelopmental impairments (Marelli et al., Reference Marelli, Miller, Marino, Jefferson and Newburger2016; Marino et al., Reference Marino, Lipkin, Newburger, Peacock, Gerdes, Gaynor, Mussatto, Uzark, Goldberg, Johnson, Li, Smith, Bellinger and Mahle2012). The neurobehavioral profile in early childhood is characterized by impairments in motor (Snookes et al., Reference Snookes, Gunn, Eldridge, Donath, Hunt, Galea and Shekerdemian2010) and language development (Fourdain et al., Reference Fourdain, St-Denis, Harvey, Birca, Carmant, Gallagher and Trudeau2019), whereas cognitive and executive function difficulties become evident at school-age (Feldmann et al., Reference Feldmann, Bataillard, Ehrler, Ullrich, Knirsch, Gosteli-Peter, Held and Latal2021). However, early detection of adverse neurodevelopment is challenging but is key for providing tailored follow-up programs and implementing early therapies (Marino et al., Reference Marino, Lipkin, Newburger, Peacock, Gerdes, Gaynor, Mussatto, Uzark, Goldberg, Johnson, Li, Smith, Bellinger and Mahle2012).
Neonatal visual performance is an early and sensitive marker of short-term neurodevelopmental outcomes in the preterm population (Ricci et al., Reference Ricci, Romeo, Gallini, Groppo, Cesarini, Pisoni, Serrao, Papacci, Contaldo, Perrino, Brogna, Bianco, Baranello, Sacco, Quintiliani, Ometto, Cilauro, Mosca, Romagnoli and Mercuri2011). In neonates with CHD, studies indicated that neonatal visual orienting is impaired (Owen et al., Reference Owen, Shevell, Donofrio, Majnemer, McCarter, Vezina, Bouyssi-Kobar, Evangelou, Freeman, Weisenfeld and Limperopoulos2014) and neonatal neurobehavior such as tone and regulation can be altered (Hogan et al., Reference Hogan, Winter, Pinto, Weng, Sheng, Conradt, Wood, Puchalski, Tani and Miller2018; Massaro et al., Reference Massaro, Glass, Brown, Chang, Krishnan, Jonas and Donofrio2011), indicating the need for early bedside assessments of neonatal neurobehavior.
Objectives
Thus, the aim of this pilot study was twofold. First, we aimed to compare neonatal visual maturity between neonates with CHD and controls using a neonatal visual assessment battery. Second, we investigated whether the assessment of neonatal visual maturity could serve as a sensitive marker for early neurodevelopmental outcomes in infants with CHD.
Methods
Study population
In this prospective observational pilot study, neonates with complex CHD undergoing cardiopulmonary bypass surgery or Giessen hybrid approach were recruited between October 2017 and May 2019 at the neonatal intensive care unit of the University Children’s Hospital Zurich. Exclusion criteria were a known genetic syndromal disorder, congenital visual impairments, or premature birth below 37 weeks of gestation. Healthy-term neonates were recruited. Parental written informed consent was obtained. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Neonatal visual assessment
Neonatal visual maturity was examined with the comprehensive assessment battery by Ricci et al. (Reference Ricci, Cesarini, Romeo, Gallini, Serrao, Groppo, De Carli, Cota, Lepore, Molle, Ratiglia, De Carolis, Mosca, Romagnoli, Guzzetta, Cowan, Ramenghi and Mercuri2008). The assessment evaluates spontaneous and targeted neonatal gaze behavior and ocular movements in response to simple standardized visual stimuli (Ricci et al., Reference Ricci, Cesarini, Romeo, Gallini, Serrao, Groppo, De Carli, Cota, Lepore, Molle, Ratiglia, De Carolis, Mosca, Romagnoli, Guzzetta, Cowan, Ramenghi and Mercuri2008). Neonates were assessed when clinically stable and in behavioral state 3–4 (Prechtl, Reference Prechtl1974). The examinations were carried out as described by Ricci et al. (Reference Ricci, Romeo, Gallini, Groppo, Cesarini, Pisoni, Serrao, Papacci, Contaldo, Perrino, Brogna, Bianco, Baranello, Sacco, Quintiliani, Ometto, Cilauro, Mosca, Romagnoli and Mercuri2011). The results were ranked on a global score from 0 to 9. Each item was scored 0 if it fell into the 90th percentile, and 1 for abnormal items if the gaze behavior fell outside the 90th percentile. Percentiles were based on a reference cohort assessed at 72 hr of life published by Ricci et al. (Reference Ricci, Cesarini, Romeo, Gallini, Serrao, Groppo, De Carli, Cota, Lepore, Molle, Ratiglia, De Carolis, Mosca, Romagnoli, Guzzetta, Cowan, Ramenghi and Mercuri2008). A summed global score >1 was considered abnormal as suggested by Ricci et al. (Reference Ricci, Romeo, Gallini, Groppo, Cesarini, Pisoni, Serrao, Papacci, Contaldo, Perrino, Brogna, Bianco, Baranello, Sacco, Quintiliani, Ometto, Cilauro, Mosca, Romagnoli and Mercuri2011). The assessment took less than 10 min. Global scores were only calculated for complete assessments.
Neurodevelopmental outcomes
Neurodevelopment was assessed at 1 year of age using the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III; Bayley, Reference Bayley2006) that provides three composite scores: cognitive, language, and motor composite score (mean 100, standard deviation ± 15).
Statistical analysis
Statistical analyses were performed using R Statistical Programming (R Core Team, 2019). The prevalence of normal versus abnormal neonatal visual scores in postoperative neonates with CHD and controls was compared using a chi-square test, and total scores were compared by means of a Mann–Whitney U test. Bayley-III composite scores were dichotomized into favorable (>85) and unfavorable (<85) neurodevelopment. To test the predictive value of the global neonatal visual assessment score for unfavorable neurodevelopment an empirical receiver operating characteristics curve was generated and the area under the curve was calculated.
Results
Twenty-seven neonates with CHD and 25 healthy controls were enrolled in the study. Clinical and demographic variables of the study population are shown in Table 1.
Table 1. Clinical and demographic variables of the study population
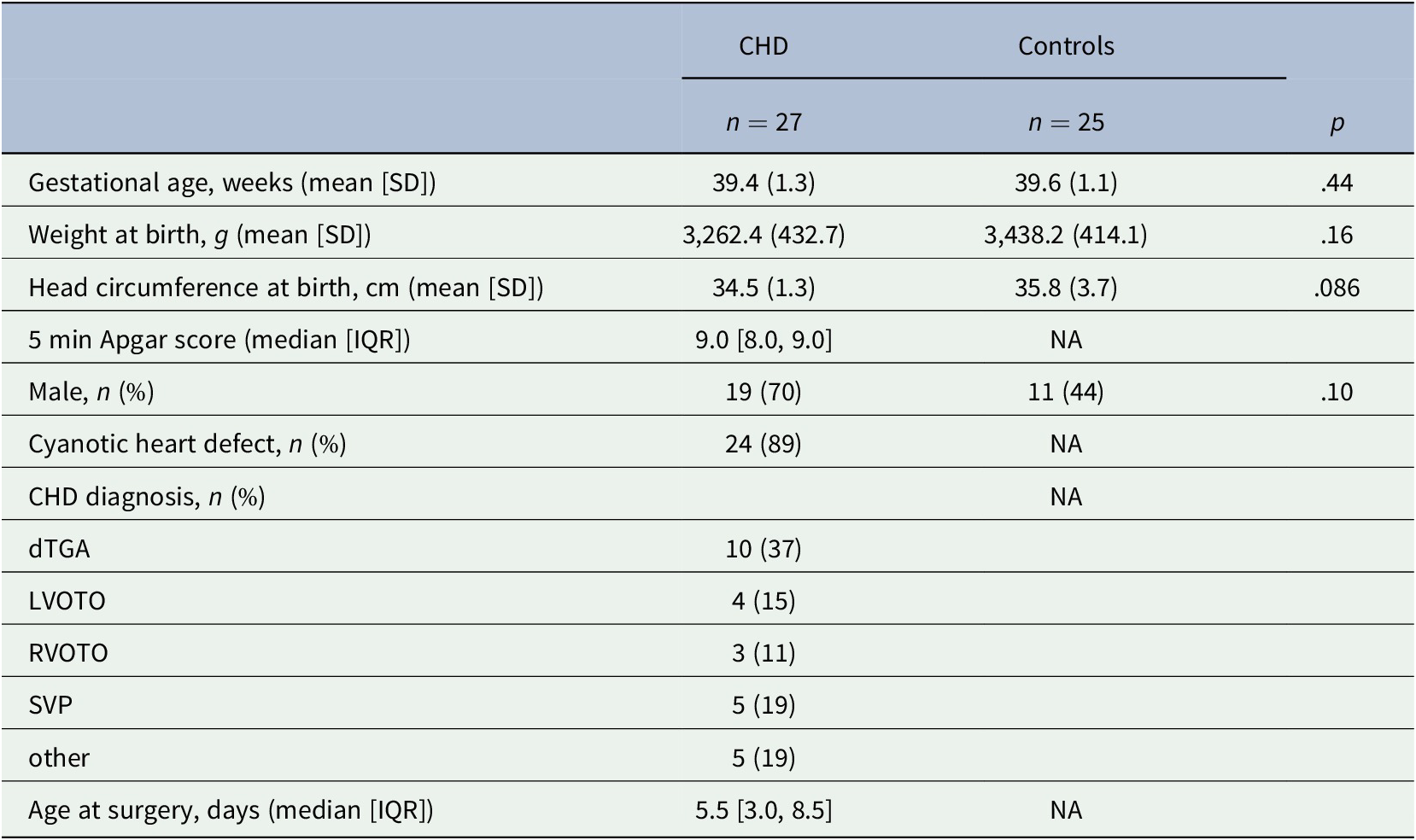
Abbreviations: CHD, congenital heart disease; dTGA, dextro-transposition of the great arteries; LVOTO, left ventricular outflow tract obstruction including aortic arch obstruction n = 3, and aortic valve stenosis n = 1; NA, not applicable/available; RVOTO, right ventricular outflow tract obstruction including pulmonary atresia n = 1 and heterotaxy syndrome n = 1; SVP, single ventricle physiology including hypoplastic left heart syndrome n = 3, heterotaxy syndrome n = 1 and double inlet left ventricle n = 1; other including truncus arteriosus communis n = 1, total anomalous pulmonary venous return n = 3 and Ebstein anomaly n = 1.
Neonatal visual assessment
Five neonates with CHD were assessed preoperatively at a median [IQR] age of 20.0 [15.0, 22.0] days of life (42.1 [40.3, 42.1] weeks postmenstrual age (PMA)) and 24 neonates with CHD were assessed postoperatively, at a median age of 27.0 [21.5, 42.0] days of life (43.8 [42.6, 44.7] PMA). Reasons for missing preoperative assessment were clinical instability, fussiness, organizational problems, or parental unease with additional examinations preoperatively (n = 22). Reasons for missing postoperative assessment were delay of surgery beyond the neonatal period (n = 3). Healthy controls underwent the neonatal visual assessment at a median age of 24.0 [15.0, 32.0] days of life (43.1 [42.1, 44.1] PMA). The neonatal visual assessment was not completed in four controls due to fussiness or sleepiness towards the end of the assessment. Because of the small number of preoperative neonatal visual assessments, only postoperative assessments were analyzed. Postoperatively, 21/24 (87.5%) neonates with CHD and 19/21 (90.5%) controls were rated as normal (95% CI −0.18–0.24, p = 1.0). Median [IQR] global visual maturity scores in infants with CHD and healthy controls were both 0 [0, 1] (95% CI −0.00028–0.00051, p = .42). The profile and proportion of abnormally evaluated total scores and subitems in both groups can be found in Figure 1.
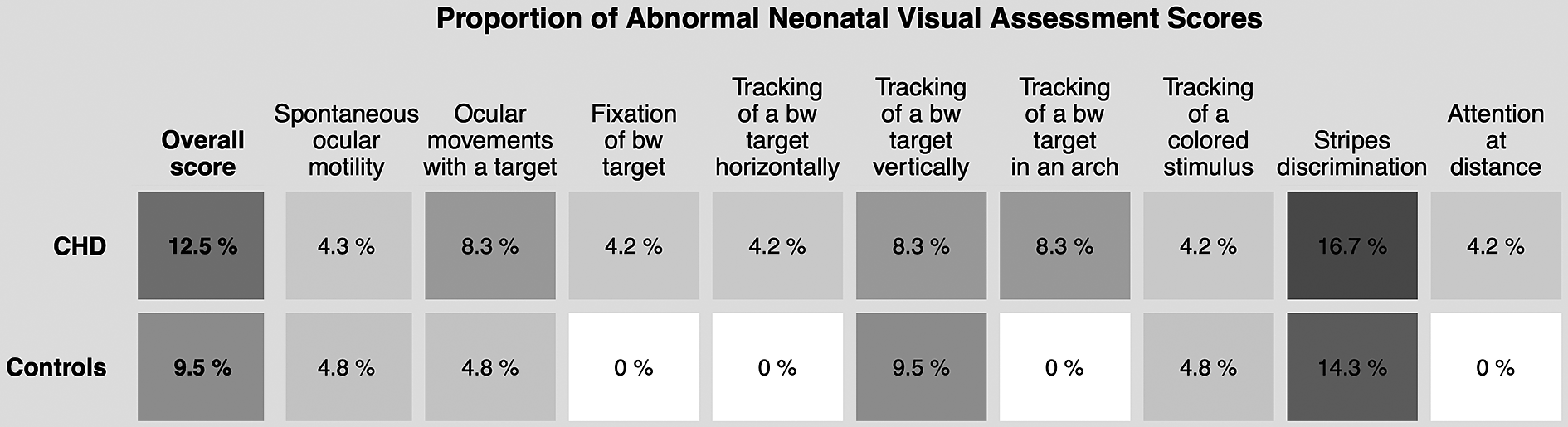
Figure 1. Proportion of abnormal overall scores and subitems in the neonatal visual assessment in postoperative neonates with CHD and healthy controls. Only complete assessments (n = 25 in CHD, n = 21 in controls) were considered. Rating of items and overall assessments were performed according to reference data and scoring system by Ricci et al. (Reference Ricci, Cesarini, Romeo, Gallini, Serrao, Groppo, De Carli, Cota, Lepore, Molle, Ratiglia, De Carolis, Mosca, Romagnoli, Guzzetta, Cowan, Ramenghi and Mercuri2008, Reference Ricci, Romeo, Gallini, Groppo, Cesarini, Pisoni, Serrao, Papacci, Contaldo, Perrino, Brogna, Bianco, Baranello, Sacco, Quintiliani, Ometto, Cilauro, Mosca, Romagnoli and Mercuri2011). Gray scale in tiles corresponds to annotated percentages. bw, black and white; CHD, congenital heart disease.
Neurodevelopmental outcomes
Twenty-two children with CHD were assessed at a median age of 13.39 (IQR 12.36, 15.93) months and 20 controls at a median age of 12.83 (IQR 12.16–13.93) months. Outcomes were missing for five children with CHD (one died, two required additional surgical interventions at 12 months of age, one was excluded due to a severe hypoxic-ischemic event, and one was lost to follow-up) and for five controls (all lost to follow-up). Among the children with CHD unfavorable outcomes were detected in 9%, 14%, and 32% of the cognitive, language, and motor domain, respectively, whereas in controls, this proportion was 0%, 0%, and 15% (Table 2). Abnormal scores on the postoperative neonatal visual assessment in infants with CHD were not predictive of unfavorable cognitive, language, or motor outcomes (Figure 2a–c).
Table 2. Neurodevelopmental outcomes assessed with the Bayley-III in children with CHD and healthy controls at 1 year of age
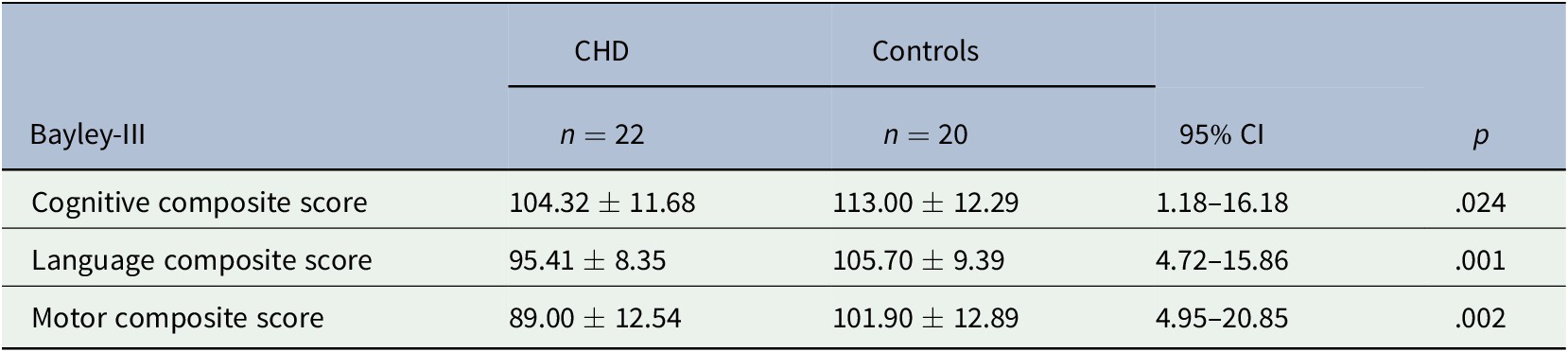
Note. Bayley-III composite score ± standard deviation.
Abbreviations: Bayley-III, Bayley scales of infant and toddler development, Third Edition; CHD, congenital heart disease; CI, confidence interval.
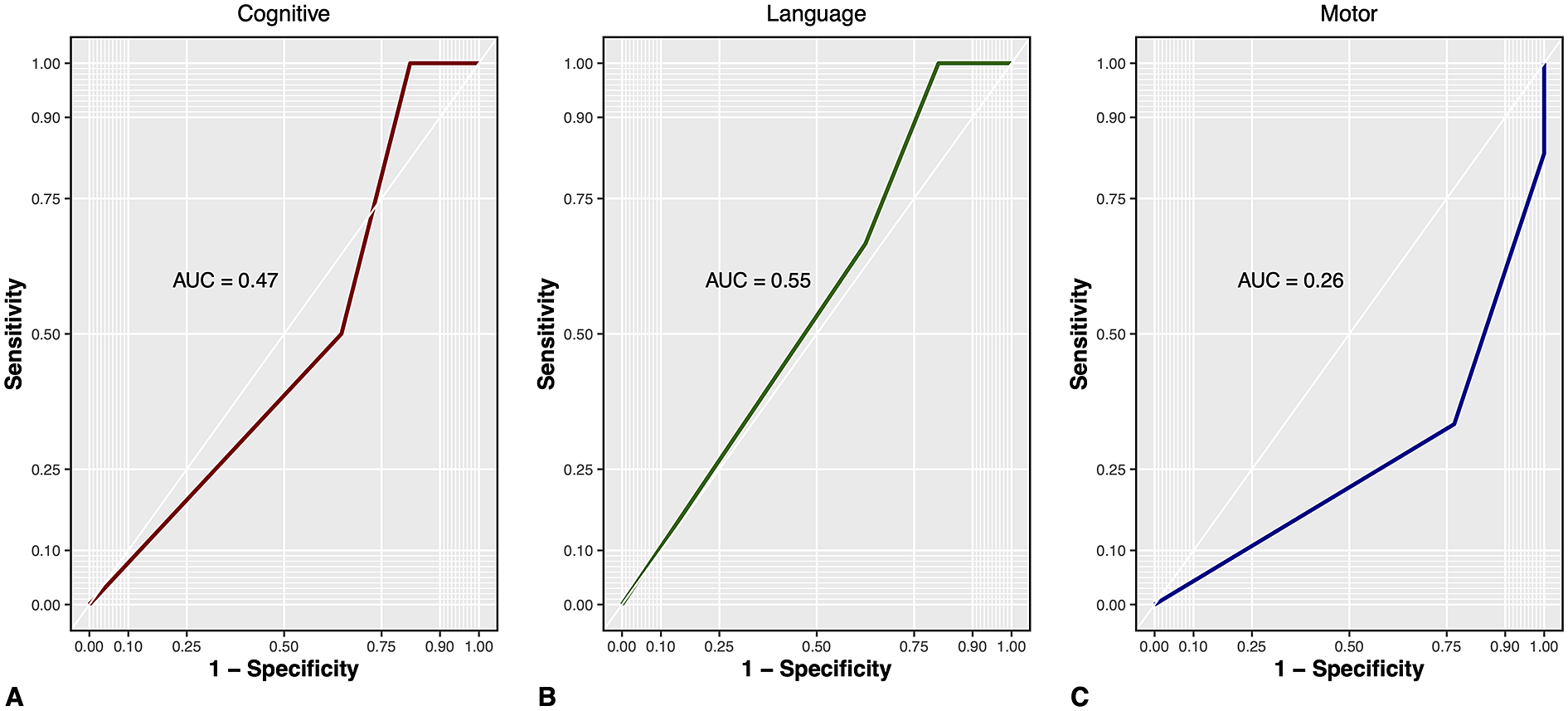
Figure 2. ROC analysis of predictive value of neonatal visual assessment for unfavorable A cognitive and B language and C motor development at 1 year of age. AUC, area under the curve; Cognitive, Bayley-III cognitive composite score; Language, Bayley-III language composite score; Motor, Bayley-III motor composite score; ROC, receiver operating characteristics curve.
Discussion
In this prospective pilot study, we found limited feasibility of applying a standardized neonatal visual assessment in the preoperative period in neonates with CHD. Reasons for missing or incomplete assessments were multifactorial, and included clinical instability, limited attention span, and irritability making it difficult to sustain visual attention across the 10 min assessment period.
In contrast, a preoperative neonatal neurobehavioral assessment with the Einstein Neonatal Neurobehavioral Assessment Scale was reported to be feasible (Limperopoulos et al., Reference Limperopoulos, Majnemer, Shevell, Rosenblatt, Rohlicek and Tchervenkov2000; Owen et al., Reference Owen, Shevell, Donofrio, Majnemer, McCarter, Vezina, Bouyssi-Kobar, Evangelou, Freeman, Weisenfeld and Limperopoulos2014). However, the subitem “visual orienting” was only a short part of the 20 min assessment that tests muscle tone, passive and active movements, primitive reflexes, as well as visual and auditory orienting. This is in contrast to the neonatal visual assessment utilized in our study which dedicates all 10 min of the assessment to the evaluation of the visual gaze behavior, thus requiring a comparatively long period of sustained visual attention. Besides the successful application of the preoperative neurobehavioral assessment, Owen et al. (Reference Owen, Shevell, Donofrio, Majnemer, McCarter, Vezina, Bouyssi-Kobar, Evangelou, Freeman, Weisenfeld and Limperopoulos2014) reported a high prevalence of neonates (65%) presenting with difficulties to stay in an alert behavioral state and a tendency towards drowsiness and irritability which corroborates our observations. Similarly, we found that the behavioral state was one of the main obstacles to successfully carry out the neonatal visual assessment, particularly in the preoperative period. However, when developing and establishing the neonatal visual assessment battery, Ricci et al. (Reference Ricci, Romeo, Gallini, Groppo, Cesarini, Pisoni, Serrao, Papacci, Contaldo, Perrino, Brogna, Bianco, Baranello, Sacco, Quintiliani, Ometto, Cilauro, Mosca, Romagnoli and Mercuri2011) were able to use their visual assessment tool in preterm born infants as early as 31 weeks of gestation even when they were still in the incubator and in full-term newborns as early as 48 hr after birth (Ricci et al., Reference Ricci, Cesarini, Romeo, Gallini, Serrao, Groppo, De Carli, Cota, Lepore, Molle, Ratiglia, De Carolis, Mosca, Romagnoli, Guzzetta, Cowan, Ramenghi and Mercuri2008). Nevertheless, neonates with CHD express a unique neurobehavioral profile compared to other at risk populations with impairments in the subdomains’ attention, need for handling, stress, and regulation (Hogan et al., Reference Hogan, Winter, Pinto, Weng, Sheng, Conradt, Wood, Puchalski, Tani and Miller2018; Massaro et al., Reference Massaro, Glass, Brown, Chang, Krishnan, Jonas and Donofrio2011).
In the postoperative period, the assessment was feasible, but no difference in visual maturity was detected between neonates with CHD and healthy controls. Both neonates with CHD and healthy controls reached the best scores in many subitems, indicating that the tool is not discriminative of the mature visual behavior at the postoperative time point. This is likely because the assessment tool should be used during the first days of life (Ricci et al., Reference Ricci, Cesarini, Romeo, Gallini, Serrao, Groppo, De Carli, Cota, Lepore, Molle, Ratiglia, De Carolis, Mosca, Romagnoli, Guzzetta, Cowan, Ramenghi and Mercuri2008) and the gaze behavior was scored by comparison to a reference population that was evaluated at 72 hr of life (Ricci et al., Reference Ricci, Cesarini, Romeo, Gallini, Serrao, Groppo, De Carli, Cota, Lepore, Molle, Ratiglia, De Carolis, Mosca, Romagnoli, Guzzetta, Cowan, Ramenghi and Mercuri2008). Thus, given the rapid development of postnatal visual behavior (Mercuri et al., Reference Mercuri, Baranello, Romeo, Cesarini and Ricci2007), the late postoperative assessment time point might have obscured potential maturational differences in neonatal visual behavior between neonates with CHD and healthy controls. This is further supported by the results of others, reporting poor visual orienting in 74% of preoperatively assessed infants with CHD which was associated with the increased cerebrospinal fluid volume on MRI (Owen et al., Reference Owen, Shevell, Donofrio, Majnemer, McCarter, Vezina, Bouyssi-Kobar, Evangelou, Freeman, Weisenfeld and Limperopoulos2014). These results indicate, that the preoperative period might be a more sensitive time point to detect maturational deficits. Consequently, our postoperative data does not allow us to make final conclusions about early neonatal visual maturity in infants with CHD. Testing higher order visual functioning, such as visual fields or fixation shifts in the postoperative period might be more sensitive to detect differences in visual maturity at this age (Mercuri et al., Reference Mercuri, Baranello, Romeo, Cesarini and Ricci2007). Furthermore, a follow up of the development of visual functioning during the first year of life could have a predictive value for later outcome, as shown in infants with hypoxic-ischemic encephalopathy (Mercuri et al., Reference Mercuri, Haataja, Guzzetta, Anker, Cowan, Rutherford, Andrew, Braddick, Cioni, Dubowitz and Atkinson1999) and preterm born infants (Atkinson et al., Reference Atkinson, Braddick, Anker, Nardini, Birtles, Rutherford, Mercuri, Dyet, Edwards and Cowan2008).
Likely, due to the ceiling effect and lack of variability when applying the assessment in the postoperative period, we could not show an association with neurodevelopmental outcomes at 1 year of age.
This study has limitations worth mentioning. This is a pilot study in a small sample of neonates with CHD. The bedside assessment of neonatal visual maturity was only performed when parental consent was obtained and neonates were clinically stable, resulting in the risk of missing eligible neonates in the brief preoperative period and a selection bias towards neonates with a less complex clinical course. When finding abnormal neonatal visual maturity in clinically ill patients, such as neonates with CHD, it is difficult to distinguish between poor performance due to a complex clinical condition or due to truly delayed visual maturation. Thus, follow up of infants with abnormal assessments is warranted.
Conclusion
In the preoperative period, applying the neonatal visual assessment in this specific sample of neonates with CHD was not feasible. In the postoperative period, the assessment was practicable, however, a delay in visual maturation and an association with later neurodevelopment was masked by the lack of sensitivity at the late testing time point. Further research is warranted to test higher order visual functioning postoperatively to further explore early visual development in neonates with CHD and assess whether it can serve as a much needed window to later neurodevelopment.
Acknowledgments
We are deeply indebted to all patients and families who participated in this study. We would like to thank the postnatal ward of the Kantonsspital Winterthur and the pediatric practice “Kind im Zentrum” of Dr. med. Sepp Holtz in Zurich for their collaboration and support in the recruitment process. Furthermore, the authors would like to extend special thanks to Daniela Ricci for providing neonatal visual assessment training and rating support.
Data availability statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Funding statement
This work was supported by the Mäxi Foundation and the Anna Müller Grocholski Foundation Zurich.
Conflict of interest
The authors have no conflicts of interest to declare.
Authorship contributions
B.L. conceived and designed the study. M.F. conducted the examinations, conducted the formal analysis, and drafted the manuscript. C.H., V.B., W.K., and B.L. helped interpret the data and revised the final manuscript.







Comments
Comments to the Author: The authors provide a well written manuscript. Some suggestion that may help are:
1) It is better to label person 1st, i.e. children with CHD throughout the paper. 2) Did the clinical demographics differ between groups? It would be helpful to add p-values in Table 1. 3) Methods: Is the visual scale valid and reliable in each group? If yes, that detail would be helpful. If not, please add that as a limitation. 4) Methods: Was a power analysis performed for the AUC analysis? If yes, please add that detail. 5) Discussion: How did your visual findings relate to other studies? Why do you think your results differed? 6) If testing visual fields/fixation shifts is more sensitive, why wasn’t this tool used? 7) Conclusion: Rewording sentence 1 in the 1st paragraph should be “specific to this sample”.