1. Introduction
Future autonomous cars will rely on computer systems, sensors, and satellite navigation which require extensive electronics that increase the vehicle’s weight (Gao et al., Reference Gao, Hensley and Zielke2014). Due to their high strength-to-weight-ratio, magnesium (Mg) structural alloys are a viable solution for vehicle-weight reduction in future cars. Mg-6wt%Al (Mg-6Al) casting alloys demonstrate the optimum level of strength and ductility for most automotive applications but further increase in their ductility will lead to their extended use in crashworthy car-body components (Friedrich & Mordike, Reference Friedrich and Mordike2006). The Mg17Al12 precipitate, the main second phase, improves strength but reduces ductility (Friedrich & Mordike, Reference Friedrich and Mordike2006; Nave et al., Reference Nave, Dahle and StJohn2000).
Studies have shown that yttrium (Y) additions can improve the ductility of Mg-Al alloys (Su et al., Reference Su, Zhang, Cheng, Liu and Cao2010; Tahreen et al., Reference Tahreen, Chen, Nouri, Li, Alderman, Manuel and Hort2016). When Y is in the solid solution of α-Mg then it can improve its mechanical properties via: (1) decrease of the Stacking Fault Energy (SFE) activating the pyramidal <c+a> disclocations (Sandlöbes et al., Reference Sandlöbes, Friák, Zaefferer, Dick, Yi, Letzig and Raabe2012) or (2) forming long period stacking ordered (LPSO) phases when a transition metal is present (Kawamura & Yamasaki, Reference Kawamura and Yamasaki2007). However, with Al present, the solubility of rare earths in α-Mg is nil, instead brittle precipitates (Pourbahari et al., Reference Pourbahari, Emamy and Mirzadeh2017; C. Wang et al., Reference Wang, Dai, Liu, Zhang and Wu2015; L. Wang et al., Reference Wang, Feng, Guo, Wang, Fu, Zhao and Cao2019) form. Researchers have observed that the modification of β-Mg17Al12 in Mg-Al-Y alloys is responsible for improved mechanical properties (Boby et al., Reference Boby, Pillai and Pai2013; Cai et al., Reference Cai, Guo, Su, Liu and Chen2018; Kashefi & Mahmudi, Reference Kashefi and Mahmudi2012; S.-R. Wang et al., Reference Wang, Guo, Yang and Wang2009). Previous work (Korgiopoulos & Pekguleryuz, Reference Korgiopoulos and Pekguleryuz2020) by the authors has shown that the refinement of β-Mg17Al12 improves the ductility of Mg-6Al cast alloys at trace Y additions. This has been attributed to co-precipitation (at close temperatures) of the Al4MgY phase with the Mg17A12 phase. Thermodynamics also predicted that the formation temperatures of the two phases deviate at higher Y levels leading to a loss in nucleant effectiveness.
2. Objective
The current work investigates the effect of Y additions (0.3wt%) to see if β-phase refinement and ductility improvement seen in trace levels of Y are also observed at higher Y levels and to elucidate the role of Y on the mechanical properties of Mg-6Al based alloys.
3. Methods
Mg-6Al-0.3Y (in wt%) alloy was synthesized using commercial purity (99.98%) Mg, 99.9% pure Al granules and 99.9% pure Y rods. The total mass of the alloying additions was 617 gr using 95% recovery factor for Al and 60% for Y. The alloying additions were made at 720 °C in a graphite crucible under CO2/SF6 protective atmosphere. A graphite crucible was used because it is affordable, and it does not react with magnesium. The cleaning after casting is efficient and leaves no residuals that could possibly contaminate the subsequent castings. The molten alloy was then poured under protective atmosphere into a preheated (400oC) steel mold to produce a flat plate. The actual composition (in wt%) of the alloy according to inductively coupled plasma atomic-emission spectroscopy is: 6.26%Al, 0.29%Y, 0.02% impurities (Fe, Mn) with Mg as balance. A scanning electron microscope (SEM-Hitachi SU3500) with an energy dispersive X-ray spectroscopy (EDS) detector was used for microstructural investigation. The grain size of α-Mg was measured with the intercept method using a Nikon-Epiphot 200 optical microscope after etching the samples with 4.2gr picric acid, 10 ml acetic acid, 10 ml distilled water and 70 ml ethanol. The ImageJ software (Rasband, Reference Rasband2011) was used to measure the precipitates and the grain size. The crystallographic information was obtained by XRD (Bruker D8 Discovery X-Ray Diffractometer-Cu source) in the bulk sample and after matrix extraction by using 5% acetic acid. The mechanical properties were evaluated by tensile and compression testing (MTS 810) at room temperature with a strain rate of 0.001s-1. Thermodynamic calculations (FactSage with FTlite database) (Bale et al., Reference Bale, Chartrand, Degterov, Eriksson, Hack, Mahfoud and Petersen2002) based on the CALPHAD method have been performed in equilibrium and non-equilibrium conditions (Scheil cooling).
4. Results and discussion
Mg-6Al-0.3Y alloy consists of partially divorced β-Mg17Al12, interdendritic Y-enriched precipitates and the Al enriched a-Mg phase as determined by EDS (Fig. 1). Y is detected in Mg-Al-Y and Mg-Al-Mn-Fe-Y precipitates. Both precipitates are closely associated with Mg17Al12 suggesting that they act as nucleation sites. The Y-enriched precipitates range from fine to coarse and present in three different morphologies, namely, spherical, square and plate-like, with the expectation that only the finer precipitates can act as refiners for the β phase. Table 1 shows the composition of the β-phase and Al2Y as per the EDS analysis. No Y was detected in the a-Mg solid solution. There is close association (Figs 1 and 2) of the Al2Y precipitates with the Mg17Al12. Similar association has been observed before by the authors (Korgiopoulos & Pekguleryuz, Reference Korgiopoulos and Pekguleryuz2020) in Mg-6%Al based alloys for lower Y additions. XRD detects (Fig. 3a) Mg-Al solid solution and β-Mg17Al12 peaks in the bulk alloy. After the matrix extraction (Fig. 3b), the matrix disappears and the peaks for Al2Y and Mg17Al12 become stronger. The peak intensity of spectra b is lower due to the low amount and small size (average size ~10-50 microns) of the extracted precipitates.
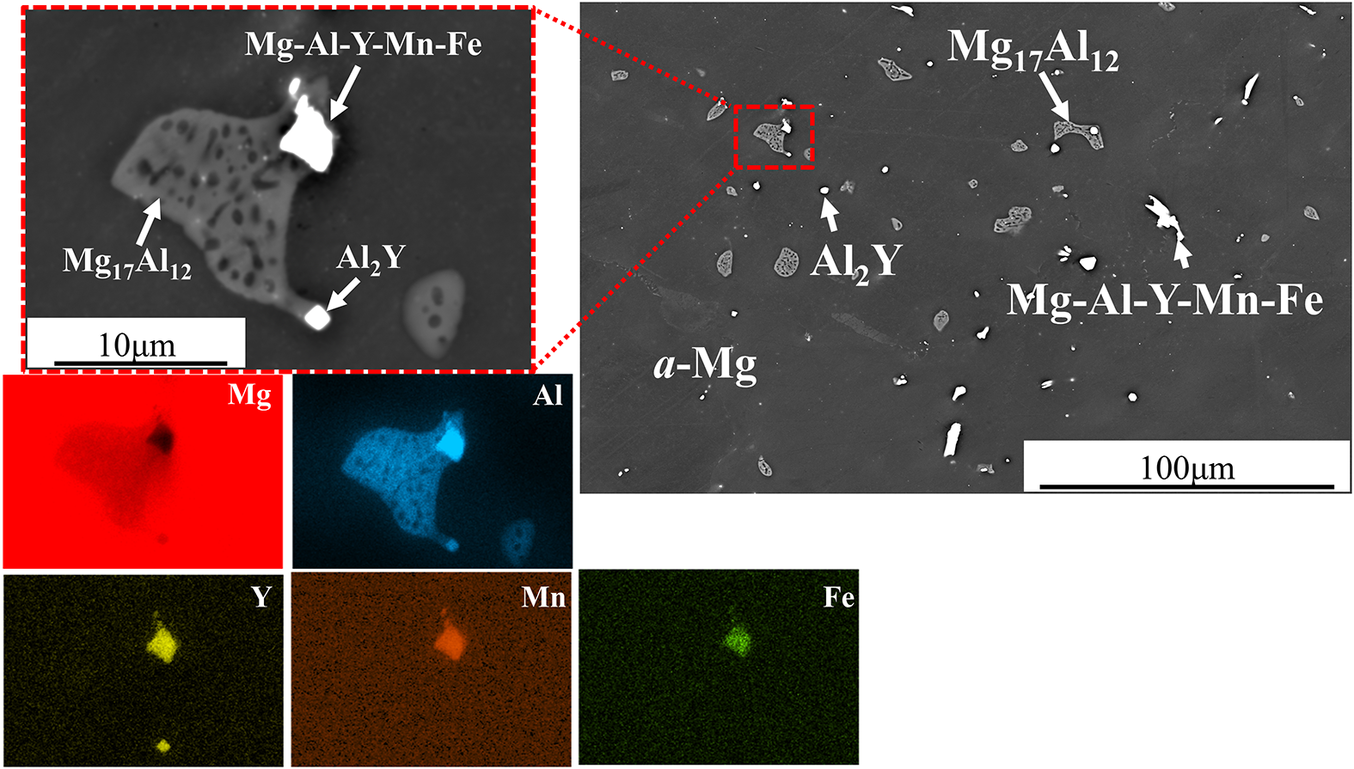
Figure 1. SEM/BSE as-cast microstructure of Mg-6Al-0.3Y and EDS maps. The red square shows the close associations of Y enriched precipitates with Mg17Al12.
Table 1. EDS on the precipitates after matrix extraction
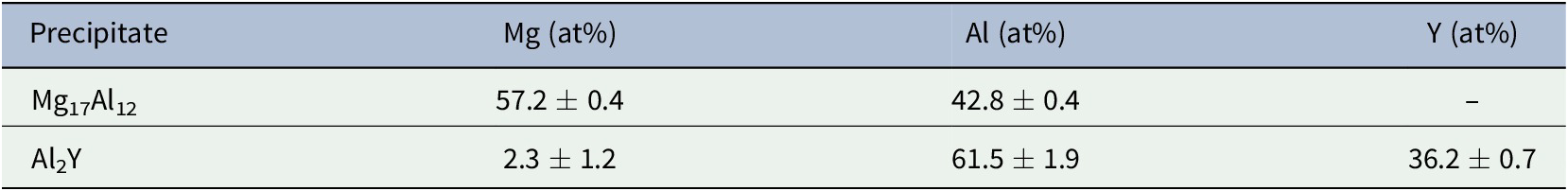
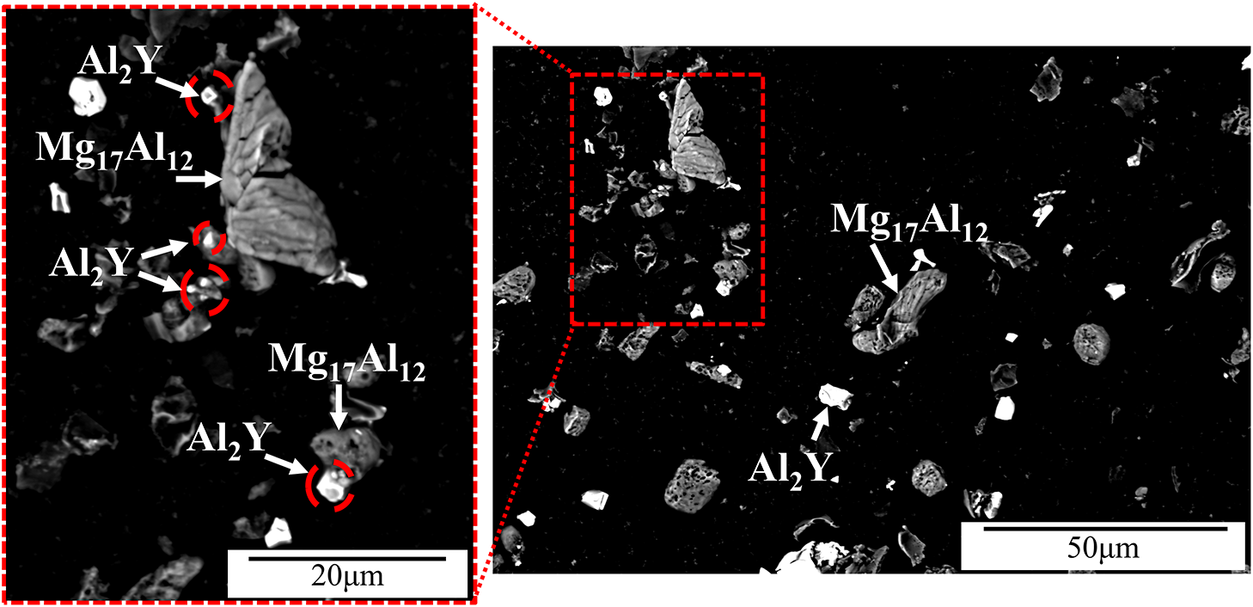
Figure 2. Mg-Al-0.3Y alloy after matrix extraction. The red circles show the Al2Y precipitates associated with the Mg17Al12 phase.
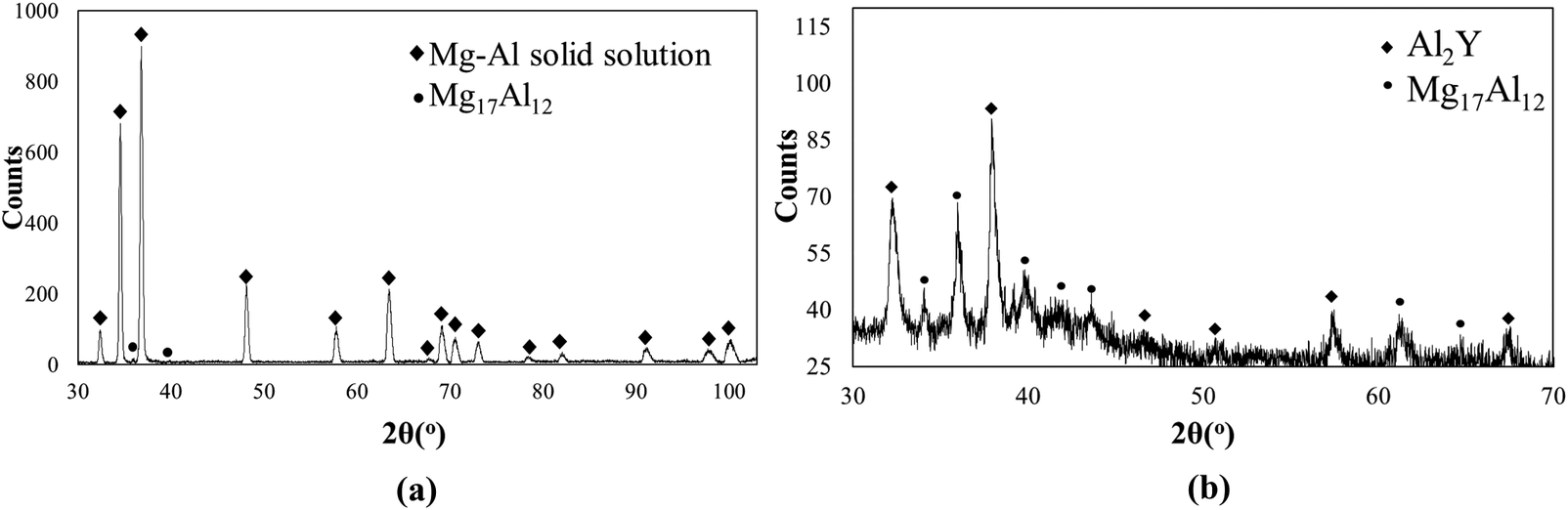
Figure 3. XRD results (a) Bulk alloy, (b) after matrix extraction.
The Mg17Al12 (Table 2) in Mg-6Al-0.3Y is 25% finer than that in the binary Mg-6Al, but 41% coarser than the alloy with trace Y amount. Additionally, high Y addition forms even coarser (~87μm2) Mg-Al-Mn-Fe-(Y) and Mg-Al-Y precipitates. The grain size of the cast Mg-6Al-0.3Y is also higher than the alloy with trace Y level and it is attributed to the coarsening of the Al2Y phase (Chang et al., Reference Chang, Qiu, Taylor, Easton and Zhang2013; Pan et al., Reference Pan, Chen, Wang, Jian and Tang2008; Zou et al., Reference Zou, Zeng, Zhai and Ding2005).
Table 2. Precipitates size and α-Mg grain size as measured with Image J
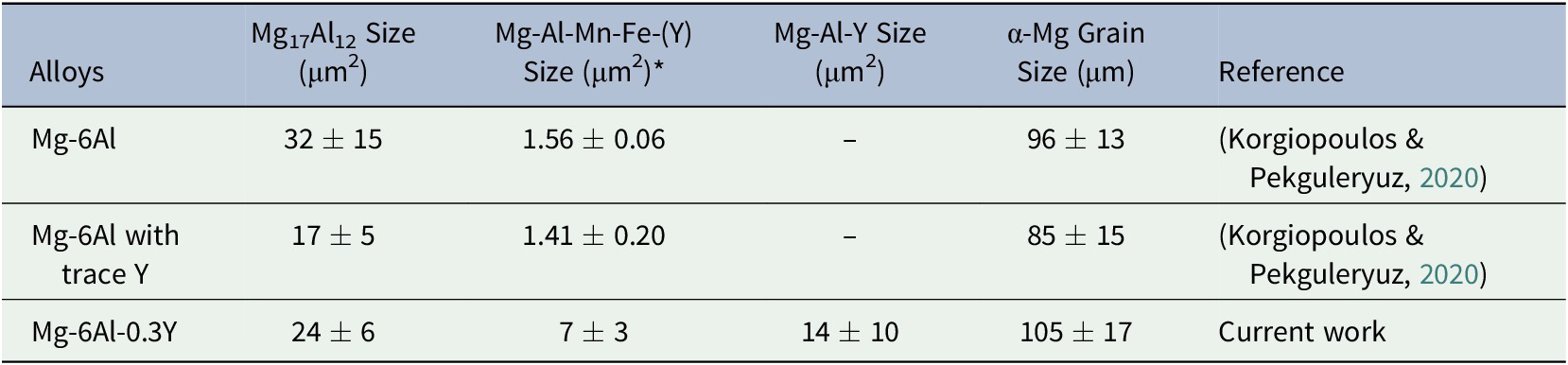
* Only precipitates in Mg-6Al-0.3Y alloy contain Y.
Mg-6Al-0.3Y has the highest tensile yield strength (YS) of the three alloys (Table 3). Compared to the Mg-Al with trace Y, the tensile ductility (% El), ultimate tensile strength (UTS), and the compressive strength are lower (Tables 3 and 4). According to thermodynamic simulations conducted by the authors (Table 5), the solubility of Y in Mg-Al-Y is practically nil. Instead, Y forms precipitates (ordered intermetallics) with Al such as Al4MgY, Al3Y and Al2Y. In Mg-Al-0.3Y, Al2Y forms earlier (at higher temperature) than the β-phase (Table 5) and has time to coarsen losing its effectiveness as a nucleant of the β-phase and embrittling the alloy.
Table 3. Tensile properties of as cast samples at room temperature
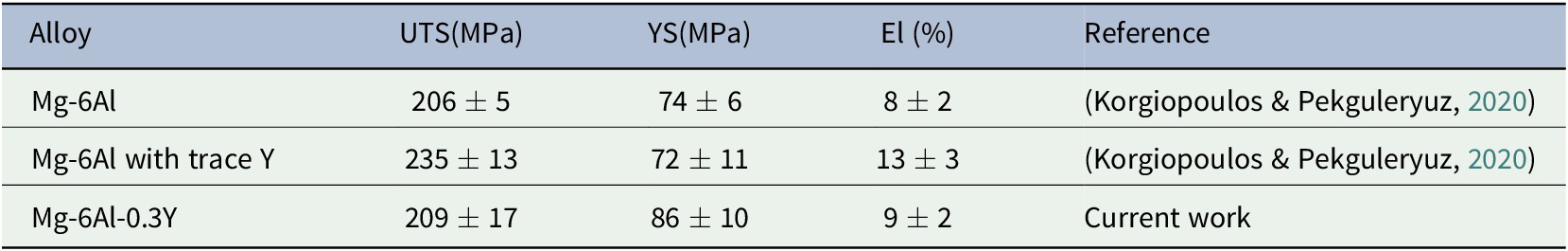
Table 4. Compression properties of as cast samples at room temperature
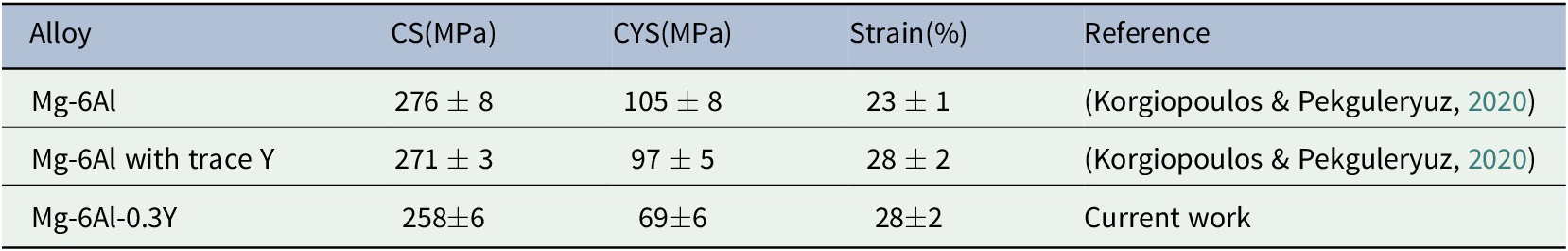
Table 5. Equilibrium and non-equilibrium (Scheil) thermodynamic calculations

5. Conclusions
The addition of 0.3wt% Y improves the tensile properties and the strain to fracture in compression of the binary cast alloys Mg-6wt%Al. The improvement in ductility and UTS is not as significant as lower (trace level) Y additions. Al2Y precipitates (determined via XRD and SEM/EDS) are in close association with the Mg17Al12 phase indicating their role as nucleants but the early formation of the Al2Y in the liquid in Mg-Al-0.3Y leads to its coarsening, resulting in some loss in mechanical properties compared to the alloy with the trace level of Y.
Funding Information and acknowledgements
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (G210358NSERCRGPIN-2016-05121). The authors thank Pierre Vermette and Dr. Amir Farkoosh for their assistance in alloy casting and discussions. Konstantinos Korgiopoulos gratefully acknowledges McGill Engineering Doctoral Award program (MEDA).
Author contributions
K. Korgiopoulos and M. Pekguleryuz conceived and designed the study. K. Korgiopoulos conducted data gathering, their analysis and interpretation. Both authors wrote the article.
Data availability statement
The raw/processed data cannot be shared at this time as the data also forms part of an ongoing study.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.











Comments
Comments to the Author: This is a useful and detailed work. The only comment which seems unjustified scientifically is the following: “These changes in mechanical properties are not due the well127 known effect of Y on SFE and LPSO since Y has negligible solubility in the a-Mg according to the thermodynamic calculations and EDS results”
I strongly advise that this comment should be changed. Although the solubility of Y in Mg is low, it is not zero. And even ppm levels of a foreign atom in any host metal can alter the SFE of some crystal planes and directions. Therefore such an effect of “low Y” levels in the system studied cannot be ruled out. And frankly speaking, this study presents no finding to justify the claim made in “Conclusions”. There exist many studies showing the effect of Y on SFEs in Mg in literature. In short, this claim is highly misleading and therefore, need be changed.
Thank you.