Published online by Cambridge University Press: 09 August 2023
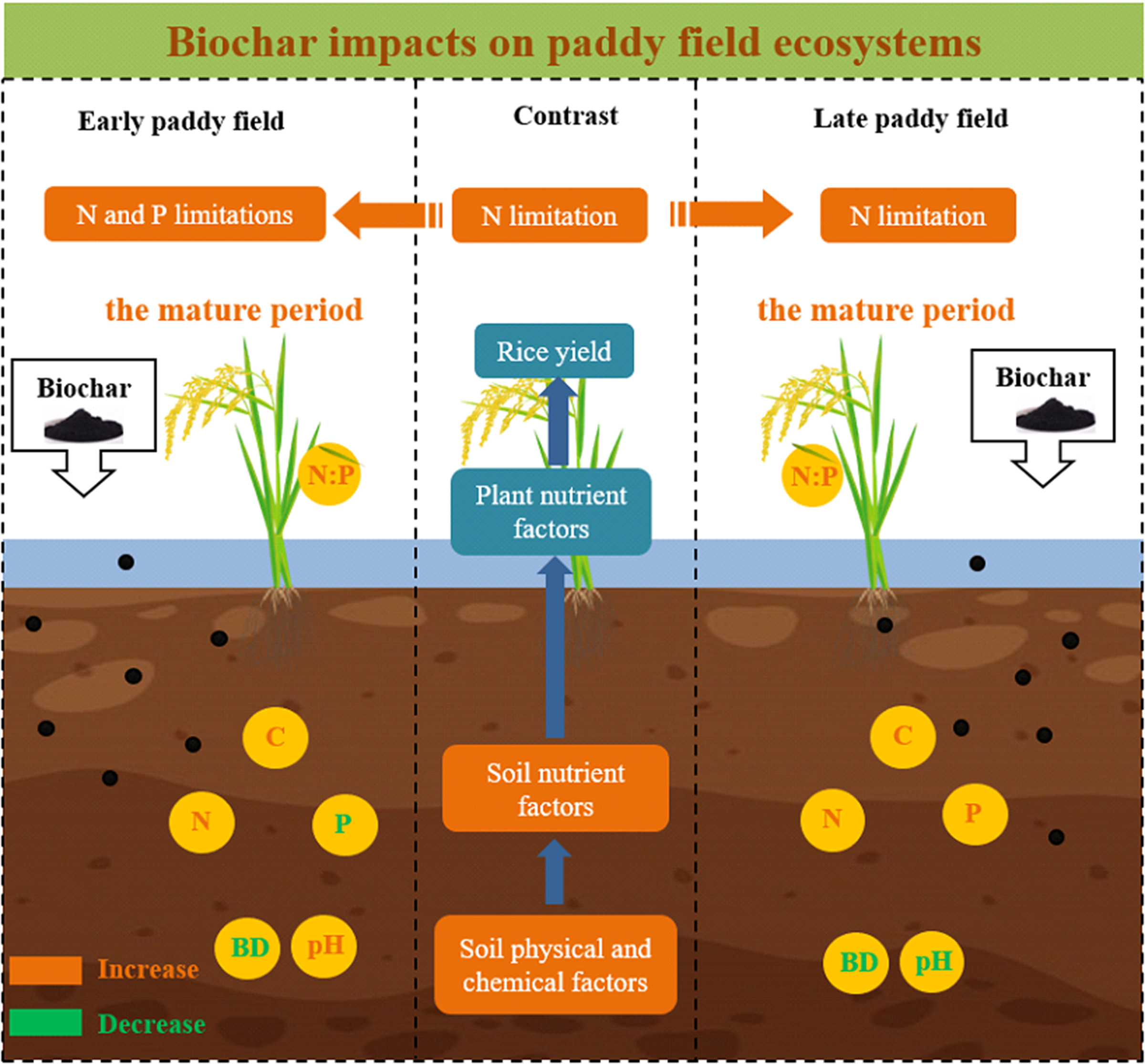
Biochar is increasingly used in crop production as a fertilizer; however, its effects on nutrient cycling and stoichiometry in rice paddy soil–plant systems are unclear. We tested for effects of contrasting rates of biochar on soil and rice plant organ carbon (C), nitrogen (N), and phosphorus (P) concentrations and stoichiometry and soil physicochemical properties in early and late paddies. Overall, biochar reduced soil bulk density by an average of 7.4%, while application at 10, 20, and 40 t ha−1 increased soil C and N concentrations in early paddies by 31.6, 41.3, and 104.2%, respectively, and by 8.0, 5.0, and 21.8%, respectively; in late paddies, there were increases of 23.0, 94.1, and 117.0%, respectively, and 6.7, 15.4, and 18.0%, respectively (P < 0.05). Following biochar application at 10, 20, and 40 t ha−1, soil concentration of P decreased in early paddies by 10.9, 19.0, and 13.9%, respectively, and increased in late paddies by 4.3, 16.4, and 20.1%, respectively. Biochar increased ratios of soil C:N and C:P in early and late paddies (P < 0.05), and there was no effect on concentration and stoichiometry of soil available nutrients. Biochar reduced rice plant organ concentration of N and P in early rice and increased leaf N:P ratios. Despite the biochar application improved nutrient status in plant–soil system, we did not observe a significant increase in yield (P > 0.05). According to the N:P value of leaves between treatments, it was found that biochar alleviated the current situation of N limitation in paddy fields during the mature period and transformed the N limitation of early rice into a joint limitation of N and P. These results show that the addition of biochar to subtropical paddy soils leads to a short-term reduction in soil bulk density and increases in soil C and N concentrations and soil fertility. Thus, biochar applied at optimal rates is likely to improve the sustainability of subtropical paddy rice production.
The original version of this article was published with an incorrect funding statement. A notice detailing this has been published and the errors rectified in the online PDF and HTML version.
Please note a has been issued for this article.