1. Introduction
Psychiatric disorders are a major public health concern. The global lifetime incidence of psychiatric disorders in adults is approximately 12.2–48.6%, with a 12-month incidence between 4.3% and 26.4% [1]. Previous studies have demonstrated that the median annual prevalence of functionally-impairing mental health conditions in children and adolescents is 25% and 12%, respectively [Reference Costello, Egger and Angold2]. In addition, psychiatric disorders are strong risk factors for suicide; approximately 70% of suicides are caused by mental illnesses [Reference Arsenault-Lapierre, Kim and Turecki3]. Psychiatric disorders are major contributors to the global disease burden; thus, the need to identify potential causal risk factors is urgent [4, 5].
Insomnia is a widespread sleep disorder with an annual incidence of approximately 35–50% in the general population [Reference Walsh, Coulouvrat, Hajak, Lakoma, Petukhova and Roth6, Reference Buysse7]. It is particularly worrisome that the prevalence of insomnia is significantly higher in psychiatric patients than in the general population [Reference Ellis, Cushing and Germain8, Reference Dolsen, Asarnow and Harvey9]. Researchers have extensively studied the relationships of sleep-related measures with psychiatric disorders. For example, Gregory et al. pointed out that the links between sleep and psychopathology are complex and likely bidirectional [Reference Gregory and Sadeh10]. Charrier and collaborators highlighted that SNPs in core circadian clock genes are associated with psychiatric disorders [Reference Charrier, Olliac, Roubertoux and Tordjman11]. Additionally, Akers and colleagues evaluated the regulation of sleep and epigenetic modifications on adult neurogenesis and memory consolidation, and suggested ways of using sleep as therapy for psychiatric disorders [Reference Akers, Chérasse, Fujita, Srinivasan, Sakurai and Sakaguchi12]. However, despite these findings, the underlying cause-effect relationships are not clearly established.
The optimal approach for determining the causal associations, randomized clinical trials (RCTs), would not have been feasible in our study because it is impractical to randomize the allocation of the participants based on their sleep status. However, estimations relying on traditional observational research are invariably subject to confounding [Reference Ioannidis, Haidich, Pappa, Pantazis, Kokori and Tektonidou13]. For example, the longstanding view that sleep problems influence the onset of certain psychiatric disorders is complemented by recent evidence suggesting that some psychiatric conditions can induce the alteration of sleep status. Reverse causality is one source of confounding; additionally, omitted variables such as education, emotional reactivity to previous events, physical activity, and alcohol dependence are another source of confounding for observational research [Reference Wang, Kwok, Au Yeung, Li, Lam and Leung14, Reference Altena, Micoulaud-Franchi, Geoffroy, Sanz-Arigita, Bioulac and Philip15]. Earlier observational studies suggested that insomnia is associated with some major psychiatric disorders [16–20]. However, several other studies have failed to provide convincing evidence to support such views [Reference Krystal21–Reference Mulraney, Giallo, Lycett, Mensah and Sciberras23]. Moreover, it is uncertain whether the associations between insomnia and psychiatric disorders are causal.
In recent years, Mendelian randomization (MR) has been widely utilized; this technique uses genetic variants (generally single-nucleotide polymorphisms, [SNPs]) as instruments to determine whether an observational association between a risk factor and an outcome is consistent with a causal effect. Since a random assortment of genotypes occurs during conception, the use of genetic variants yields a random distribution in a population, which can be compared with a conventional RCT [Reference Emdin, Khera and Kathiresan24]. The invariant nature of the DNA sequence and the unidirectional flow of biological information make MR much less susceptible to confounding factors [Reference Katan25]. The availability of summary statistics for a large number of phenotypes from genome-wide association studies (GWASs) allows for the straightforward application of summary data-based MR, especially in a two-sample design.
Therefore, in the present study, we utilized two-sample summary data-based MR to examine the causal relationships of insomnia with five major psychiatric disorders: attention deficit hyperactivity disorder (ADHD), autism spectrum disorder (ASD), major depressive disorder (MDD), schizophrenia (SCZ), and bipolar disorder (BIP).
2. Methods
2.1. Study sample
Summary statistics for insomnia were acquired from the most recent publicly-released GWAS, which comprised >10 million genetic variants in 109,402 cases and 277,131 controls from the UK Biobank [Reference Jansen, Watanabe, Stringer, Skene, Bryois and Hammerschlag26]. Insomnia cases were diagnosed using the question: “Do you have trouble falling asleep at night or do you wake up in the middle of the night?” with five response options: “I don’t know”, “never/rarely”, “sometimes”, “usually”, and “prefer not to answer”. Specifically, insomniacs were defined as participants who answered “usually”, while participants who answered “never/rarely” or “sometimes” were defined as controls [Reference Jansen, Watanabe, Stringer, Skene, Bryois and Hammerschlag26].
Summary statistics for psychiatric disorders were acquired from the Psychiatric Genomics Consortium (PGC; http://www.med.unc.edu/pgc/), which is the largest biological experiment consortium in the history of psychiatry [27]. We selected non-sex-stratified summary statistics; in addition, as two-sample summary data-based MR assumes independent samples between exposure and outcome, the psychiatric phenotypes that shared significant overlapping cohorts with insomnia were excluded (i.e., use of UK Biobank as the main cohort in the analysis). Following these standards, five most recent GWASs, one each corresponding to ADHD, ASD, MDD, SCZ, and BIP, were selected as study cohorts (Table 1).
Summary statistics for ADHD were obtained from a GWAS meta-analysis of 12 cohorts aggregated by Integrative Psychiatric Research (iPSYCH) and the PGC [Reference Demontis, Walters, Martin, Mattheisen, Als and Agerbo28]. Patients with ADHD in iPSYCH were diagnosed in accordance with criteria from the 10th revision of the International Classification of Diseases (ICD-10) (F90.0). Designs for PGC cohorts were described in the original articles [Reference Demontis, Walters, Martin, Mattheisen, Als and Agerbo28]. Summary statistics for ASD were obtained from a meta-analysis of GWASs involving 14 independent cohorts implemented by the Autism Spectrum Disorders Working Group of the PGC. Patients with ASD were diagnosed in accordance with either Autism Diagnostic Interview-Revised or Autism Diagnostic Observation Schedule criteria [29]. Summary statistics for MDD were obtained from a mega-analysis of GWASs for nine samples implemented by the Major Depressive Disorder Working Group of the PGC. Patients were assessed with validated instruments and met Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) criteria for lifetime MDD [30]. Summary statistics for SCZ and BIP were obtained from GWASs of large collections of genotyped samples implemented by the Bipolar Disorder and Schizophrenia Working Group of the PGC [31]. Patients with SCZ were diagnosed mainly via a research-based questionnaire covering the assessment protocol and associated quality control procedures [32]. Patients with BIP were diagnosed using structured diagnostic instruments based on international consensus criteria (DSM-IV, ICD-9, or ICD-10) for a lifetime diagnosis [Reference Stahl, Breen, Forstner, McQuillin, Ripke and Trubetskoy33].
The vast majority of the cohorts in each of the GWASs were from European ancestry; less than 0.1% of the participants were from non-European ancestry. Corresponding details of the studies have been reported in the cited articles; ethical approval and informed consent were obtained in the original studies.
2.2. Statistical analysis
We utilized the generalized summary data-based MR (GSMR) method developed by Zhu et al. to perform the causality estimations [Reference Zhu, Zheng, Zhang, Wu, Trzaskowski and Maier34]. Using GSMR, we calculated the ratio estimates for each SNP instrument and integrated them via the generalized least squares approach. This approach provides more power than traditional summary data-based MR methodologies because it accounts for the sampling variation in the estimated effects of the instruments both on the exposure and outcome, while other approaches assume that the effect of the instrument on the exposure is estimated without error [Reference Burgess, Butterworth and Thompson35, Reference Burgess, Dudbridge and Thompson36]. In addition, GSMR accounts for linkage disequilibrium (LD) between the SNPs, which is another confounding source in MR analysis, while other methods assume independence among the instruments [Reference Aissani37].
Table 1 Five psychiatric disorders used in this study.

The first assumption of MR is that the instruments are associated with the risk factor [Reference Burgess and Thompson38]. Thus, genome-wide statistically significant (p < 5 × 10−8) SNPs associated with insomnia were selected for preliminary instruments. To avoid strong co-linearity between the candidate SNPs, we excluded SNPs that were in LD using a criterion of r2 ≥ 0.1, and only considered the SNPs with the strongest effect on the traits for use as instruments. The remaining LD between the instruments could be resolved using the GSMR method [Reference Zhu, Zheng, Zhang, Wu, Trzaskowski and Maier34]. The individual-level genotypes of the 1000 Genomes Project Phase 3 datasets were utilized as the reference sample to estimate LD between the SNPs [39].
The second and third assumptions of MR are that the instruments are independent of the confounders between risk factors and diseases and that they influence the outcome only through risk factors. These two assumptions are collectively known as the no-pleiotropy assumption [Reference Emdin, Khera and Kathiresan24]. Pleiotropy refers to a genetic variant that affects outcomes via pathways other than a risk factor. Pleiotropic instruments often result in an inflated test statistic in MR analysis, thus biasing the estimate [Reference Hackinger and Zeggini40]. For this consideration, a heterogeneity in dependent instrument (HEIDI) analysis was utilized to remove pleiotropic SNP instruments [Reference Zhu, Zhang, Hu, Bakshi, Robinson and Powell41], and MR-Egger regression was used to verify the validity of the screened instruments with a significant deviation of the intercept from 0 considered indicative of pleiotropy in the instruments [Reference Bowden, Davey Smith and Burgess42].
To explore whether the five psychiatric disorders have any causal impact on the risk of insomnia, we also implemented reverse MR analysis (i.e., five psychiatric disorders as the exposure and insomnia as the outcome), using SNPs that are associated with psychiatric disorders as instruments. As GSMR requires a minimum of 10 instruments to be included in the analysis, a suitable relaxation of the significance threshold was used for the initial selection of instruments in some traits to allow for a sufficient number of SNPs to be included in the analysis [Reference Savage, Jansen, Stringer, Watanabe, Bryois and de Leeuw43]. Specifically, the significance thresholds for selecting instruments were relaxed to p < 5 × 10−6 for ASD and BIP, and p < 5 × 10−5 for MDD. These thresholds were determined as a trade-off between having enough SNPs for GSMR and the strength of each instrument [Reference Burgess, Scott, Timpson, Davey Smith, Thompson and EPIC- InterAct Consortium44]. The thresholds were imposed to include the SNPs most significantly associated with the traits as candidate instruments while simultaneously ensuring a minimum of 10 SNPs for each of the three models [Reference Zhu, Zheng, Zhang, Wu, Trzaskowski and Maier34, Reference Swerdlow, Kuchenbaecker, Shah, Sofat, Holmes and White45]. Additionally, we performed sensitivity analysis to assess the robustness of our findings using three other MR-based methods: inverse-variance weighting (IVW), simple median estimator (SME), and weighted median estimator (WME).
A conservative Bonferroni-corrected significance threshold of 0.005 (0.05/10, to correct for 10 tests: five for the causality of insomnia with psychiatric disorders and five for reverse causality) was used for GSMR and sensitivity analyses, and the threshold of significance for other tests was 0.05. The GCTA software package (GCTA 1.91.7; http://cnsgenomics.com/software/gcta) was used to extract the genotype data of the SNP instruments from the reference sample [Reference Yang, Lee, Goddard and Visscher46]. MR analyses were performed using R version 3.5.0 (https://www.r-project.org) and the related packages (GSMR, MendelianRandomization) [Reference R Core Team47].
3. Results
3.1. Causal effects of insomnia on psychiatric disorders
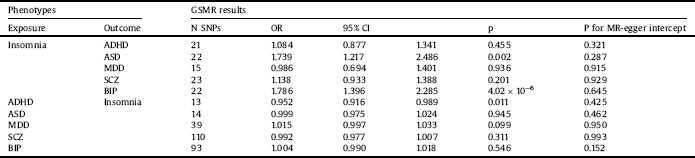
After excluding SNPs that were not available from the GWAS summary statistics or showed evidence of pleiotropy, 15 to 23 genome-wide SNPs significantly associated with insomnia were included as instruments (Table 2).
The HEIDI-outlier test suggested two SNP outliers in the instruments for the causal estimation of insomnia with ADHD. After excluding outlier SNPs, the MR-Egger intercept test provided no evidence of pleiotropy for the retained instruments (intercept p = 0.321). The estimation was not significant (OR: 1.084, p = 0.455). Hence, there was no evidence to identify the causal role of insomnia in ADHD (Table 2).
For the causal estimation of insomnia with ASD, HEIDI-outlier analysis detected one pleiotropic SNP instrument. After filtering the outlier SNP, we formed the final instrumental variable set with no evidence of pleiotropy (intercept p = 0.287 for MR-Egger). Estimates from the analyses were significant (OR: 1.739, p = 0.002), indicating that insomnia was putatively causal for ASD (Table 2).
No evidence of pleiotropy was observed based on the results of both the HEIDI-outlier analysis and MR-Egger tests (intercept p = 0.915) for the causal estimation of insomnia with MDD. However, we observed no significant effect of insomnia on MDD (OR: 0.986, p = 0.936) (Table 2).
For the analysis of the causal effect of insomnia on SCZ, evidence for pleiotropy was observed in five SNPs. Removal of the outlier SNPs eliminated the pleiotropy, and MR-Egger tests yielded a nonsignificant intercept (p = 0.929). GSMR yielded a nonsignificant effect (OR: 1.138, p = 0.201), indicating that insomnia does not causally impact the risk of SCZ (Table 2).
Following the HEIDI-outlier analysis for the causal effect of insomnia on BIP, we removed five SNPs that exhibited pleiotropy. MR-Egger test indicated no pleiotropic bias for the remaining instruments (intercept p = 0.645). GSMR yielded a significant OR of 1.786 (p = 4.02 × 10−6), suggesting that insomnia has a causal effect on BIP (Table 2).
Table 2 Bidirectional causal estimates for insomnia and psychiatric disorders by GSMR.

Abbreviations: ADHD: attention-deficit/hyperactivity disorder; ASD: autism spectrum disorder; MDD: major depressive disorder; SCZ: schizophrenia, BIP: bipolar disorder; GSMR: generalized summary data-based Mendelian randomization; N SNPs: number of SNPs retained and used in the GSMR analysis after filtered by HEIDI outlier analysis; OR: odds ratio; CI: confidence interval, p: p-value for the causal estimates; p for MR-egger intercept: p-value for intercept of MR-egger regression.
3.2. Causal effects of psychiatric disorders on insomnia
Reverse MR analysis was implemented to investigate the causal effects of five psychiatric disorders on insomnia. After the filtration of pleiotropic instruments with HEIDI-outlier analysis, a total of 13 to 110 SNPs were retained in the final instrument sets. None of the MR-Egger intercepts significantly deviated from zero, with p values of 0.425 for ADHD, 0.462 for ASD, 0.950 for MDD, 0.993 for SCZ, and 0.152 for BIP. Thus, no evidence of pleiotropy was observed after excluding the outlier SNPs (Table 2).
Reverse MR testing was only suggestive of a nominally significant causal effect of ADHD on insomnia (OR: 0.952, p = 0.011), albeit not significant after adjusting for multiple testing. The respective effects estimated from reverse GSMR analyses were 0.999 for ASD (p = 0.945), 1.015 for MDD (p = 0.099), 0.992 for SCZ (p = 0.311), and 1.004 for BIP (p = 0.546). Therefore, no evidence of reverse causality was observed between ASD, MDD, SCZ, or BIP and insomnia risk (Table 2).
3.3. Sensitivity analysis
For comparison, three other MR methods were used to detect the bi-directional causality between insomnia and psychiatric disorders. The SNP instruments used for these three methods were the same as those used for the GSMR analysis. IVW provided a suggestive causal relationship of insomnia with BIP (OR: 1.527, p = 0.019), and of ADHD with insomnia (OR: 0.933, p = 0.016). However, these suggested relationships did not survive multiple testing corrections. Using SME and WME, null estimates were obtained for the causal effects of insomnia on psychiatric disorders and the reverse causality of psychiatric disorders on insomnia (Table 3).
4. Discussion
In the present study, we utilized a GSMR model to investigate the bidirectional causal relationships between insomnia and five major psychiatric disorders. Specifically, insomnia exhibited a causal impact on ASD and BIP, while nonsignificant effects of these two disorders were observed on the risk of insomnia. No significant bidirectional associations were observed between insomnia and any of the remaining three psychiatric disorders. Notably, under the MR framework, the estimated causalities are the effects of a specific genetic component of insomnia on psychiatric disorders [Reference Zhu, Zheng, Zhang, Wu, Trzaskowski and Maier34]. Hence, it is possible that the variation in insomnia that determined by other residual components or operating through other biological mechanisms could have different causal relationships with psychiatric outcomes [Reference Böckerman, Cawley, Viinikainen, Lehtimäki, Rovio and Seppälä48].
Although some studies have reported associations between insomnia/sleep status and mental illness, they have yielded mixed results. This is due in part to the limitations inherent to observational studies. Understanding whether an association is potentially causal or if it results from confounding is generally difficult using traditional observational studies. However, the MR methodology utilized in the present study—which uses genetic variants to mimic randomization—is far less prone to confounding factors and hence provides more robust estimates.
Our work builds on proof-of-principle validation for mechanisms that may link sleep-related factors to psychiatric disorders. Briefly, several neurobiological abnormalities are common in both sleep and psychiatric disorders, including activation of the hypothalamic–pituitary–adrenal (HPA) axis, alterations in serotonin system function, and elevated production of some immune system peptides. These shared abnormalities may explain why sleep disorders are connected to the risk of developing a wide range of psychiatric disorders [Reference Krystal49]. Furthermore, the biological clock network enables the body to adapt to environmental changes by controlling circadian rhythms and regulating the expression of downstream genes [Reference Brancaccio, Edwards, Patton, Smyllie, Chesham and Maywood50]. Sleep quality impacts cognition, emotion, learning, and long-term memory, as well as the ability to adapt to the environment. An inferior sleep status, including insomnia, may cause imbalances between the internal and external environment, eventually increasing the risk of developing psychiatric disorders [Reference Charrier, Olliac, Roubertoux and Tordjman11]. Previous studies have indicated that regulating sleep via related intracellular pathways may achieve a balance in neural excitability for patients with psychiatric disorders [Reference Shi and Ueda51].
Our results indicate that insomnia causally affects ASD, supporting the results of previous studies that have highlighted the correlation between sleep disorders and ASD. Children with ASD generally experience sleep problems, with a prevalence ranging from 50 to 80% [Reference Couturier, Speechley, Steele, Norman, Stringer and Nicolson52–Reference Richdale54]; moreover, among the sleep problems observed in children with ASD, insomnia is the most common [Reference Richdale and Schreck55]. In terms of mechanisms, some studies have indicated that polygenic variations in the circadian rhythm and clock genes may explain the development of ASD [Reference Glickman56]. Other studies have highlighted the significance of alterations in HPA axis function and cortisol secretion in insomnia mechanisms, which may further trigger the development of ASD [Reference Tomarken, Han and Corbett57, Reference Sharpley, Bitsika, Andronicos and Agnew58].
We also observed a significant causal effect of insomnia on BIP, providing evidence to support a biopsychosocial framework between these two traits. Previous studies have demonstrated that patients with BIP often experience a range of sleep abnormalities, and insomnia is a common feature among them [Reference Geoffroy, Scott, Boudebesse, Lajnef, Henry and Leboyer59, Reference Harvey, Schmidt, Scarna, Semler and Goodwin60]. Moreover, insomnia may confer an increased risk for the subsequent onset and recurrence of BIP [Reference Ritter, Hofler, Wittchen, Lieb, Bauer and Pfennig61, Reference Etain, Godin, Boudebesse, Aubin, Azorin and Bellivier62]. One possible mechanism underlying this causal relationship is that insomnia is related to difficulties in some areas of emotion regulation and consecutive emotional reactivity, and dysregulation of emotion may greatly affect symptoms of BIP [Reference Altena, Micoulaud-Franchi, Geoffroy, Sanz-Arigita, Bioulac and Philip15, Reference Palagini, Cipollone, Masci, Caruso, Paolilli and Perugi63]. Another study reported that gamma-aminobutyric acid (GABA) levels are decreased in patients with BIP. This may explain the course of BIP, as GABA is known to inhibit brain activity during sleep [Reference Saper, Scammell and Lu64].
Table 3 Bidirectional causal estimates for insomnia and psychiatric disorders by three other MR methods.

Abbreviations: ADHD: attention-deficit/hyperactivity disorder; ASD: autism spectrum disorder; MDD: major depressive disorder; SCZ: schizophrenia, BIP: bipolar disorder; IVW: inverse-variance weighting; SME: simple median estimator; WME: weighted median estimator; OR: odds ratio; CI: confidence interval, p: p-value for the causal estimates.
Our study highlighted the importance of treating insomnia and related sleep disorders to improve psychiatric disorders and overall public health. Recently, an RCT of participants with chronic insomnia verified the efficacy and availability of web-based cognitive behavioral therapy for insomnia intervention [Reference Ritterband, Thorndike, Ingersoll, Lord, Gonder-Frederick and Frederick65]. In another study, the authors proposed a cross-platform, just-in-time adaptive intervention for the treatment of insomnia symptoms [Reference Pulantara, Germain, Parmanto, Richardson, Khan and Rode66]. Such insomnia interventions are of great public health and societal importance, as they may further prevent the long-term effects of insomnia on mental health.
The current study has several strengths. First, the use of the MR approach allows one to control for unmeasured confounders and reverse causation, thus providing opportunities for reliable causal inference within the framework of observational research and allowing for the generation of hypotheses concerning the direction of causation between heritable variables [Reference Swerdlow, Kuchenbaecker, Shah, Sofat, Holmes and White45]. In addition, the power of summary data-based MR analysis can be greatly improved by exploiting large sample sizes; thus, the effect estimates identified in our study are more powerful than the individual data-based analyses of small studies [Reference Burgess, Butterworth and Thompson35]. Second, the GSMR method offers more power than other comparative MR methods in the sensitivity analysis, as these other methods do not account for error in the SNP–exposure association estimates or for correlations between SNPs—a factor that is especially important when the number of instruments is large [Reference Zhu, Zheng, Zhang, Wu, Trzaskowski and Maier34, Reference Swerdlow, Kuchenbaecker, Shah, Sofat, Holmes and White45]. Additionally, pleiotropic instrument filtration via HEIDI-outlier analysis provided more robust results. Third, although the disease data we applied in the study were acquired from case-control studies, the estimated causative effect of exposure on the outcome can be interpreted as that in the general population, rather than that in the disease population only, using GSMR [Reference Zhu, Zheng, Zhang, Wu, Trzaskowski and Maier34].
However, our study should be interpreted in the context of the following limitations. First, the lack of evidence for the causative effects of some psychiatric disorders on insomnia may be attributable to weak instrument bias. The numbers of genome-wide significant variants associated with some psychiatric disorders were not sufficient for GSMR. Thus, we relaxed the significance threshold during instrument selection, which means that some SNPs were not as strongly associated with the traits. The weak instrument bias results in the direction of the null; such bias may explain the lack of a causal relationship between these psychiatric disorders and insomnia [Reference Burgess, Scott, Timpson, Davey Smith, Thompson and EPIC- InterAct Consortium44]. Therefore, these analyses should be revisited when more genome-wide significant SNPs become available from future GWASs. Second, two-sample MR strategies including GSMR only allow for the examination of linear relationships between exposure and outcome, which we assumed in the present study due to the limited information that we could acquire from GWAS summary data. Also, we could not investigate gender-or age-specific effects because of the lack of data from gender- or age-stratified analyses; further research is warranted when future stratified GWAS summary data is available.
In conclusion, the present study provides evidence for the causal effects of insomnia on ASD and BIP. These results offer genetic support for previous observational associations between insomnia and psychiatric disorders. Such findings may aid clinicians and researchers in the development of novel intervention approaches, as well as in performing in-depth explorations of mechanisms of related psychiatric disorders underlying these causal associations.
Psychiatric disorders lead to an impaired quality of life and heavy social burdens; thus, exploring the associated risk factors of psychiatric problems has clinical and public health priorities. Our findings imply that insomnia increases the risk of certain psychiatric diseases; this finding may guide further work toward exploring effective therapeutic strategies. These results further emphasize the need for thoughtful applications of behavioral and pharmacological therapy to reform insomnia symptoms because of its downstream consequences on mental health, and provide evidence of including insomnia as part of the pragmatic diagnostic criteria for related psychiatric disorders.
Declarations of interest
None.
Acknowledgments
Authors sincerely acknowledge the original GWASs and the related consortiums for the collection and management of the large-scale data resources. Authors also thank the research funders. This work was supported by the National Natural Science Foundation of China [grant number: 81872715] and the Innovation Foundation for Graduate Student of Shanxi Province [grant number: 2018JD25]. We declare that the funders had no role in study design, in the collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.





Comments
No Comments have been published for this article.