Introduction
The extreme weight condition (EWC) construct has been used to classify individuals with unhealthy eating behaviors, altered body adiposity, metabolism, and nutrition patterns [Reference Fagundo, de la Torre, Jiménez-Murcia, Agüera, Granero and Tárrega1–Reference Cuzzolaro3]. These clinical conditions would be distributed within a continuum where, at one extreme anorexia nervosa (AN) is found, whereas the other end is represented by obesity [Reference Fagundo, de la Torre, Jiménez-Murcia, Agüera, Granero and Tárrega1–Reference Cuzzolaro3]. While AN is an eating disorder (ED) characterized by a low body mass index (BMI) (i.e., BMI < 18 kg/m2), obesity is defined as a metabolic disorder with a BMI ≥ 30 kg/m2, according to the World Health Organization [4]. EDs are mental illnesses with multifactorial etiopathogenesis involving biological to psychosocial factors [5, Reference Treasure, Duarte and Schmidt6]. Bulimia nervosa (BN) and binge eating disorder (BED) are also EDs, which could be understood under the umbrella of the so-called binge spectrum disorders (BSDs) [Reference Miranda-Olivos, Testa, Lucas, Sánchez, Sánchez-González and Granero7], with an important lifetime prevalence of obesity [Reference Agüera, Lozano-Madrid, Mallorquí-Bagué, Jiménez-Murcia, Menchón and Fernández-Aranda8–Reference Giel, Bulik, Fernandez-Aranda, Hay, Keski-Rahkonen and Schag10]. In fact, the frequency of binge eating episodes (BEs) can increase the risk for obesity in almost half of the individuals with BSD [Reference Agüera, Lozano-Madrid, Mallorquí-Bagué, Jiménez-Murcia, Menchón and Fernández-Aranda8, Reference Villarejo, Fernández-Aranda, Jiménez-Murcia, Peñas-Lledó, Granero and Penelo9], suggesting the existence of shared biological and environmental vulnerability factors between both entities [Reference Bulik, Sullivan and Kendler11–Reference Volkow, Wang, Fowler, Tomasi and Baler16].
In the last decades, the endocannabinoid (eCB) system has emerged as a biological factor implicated in the pathogenesis of EWC, given its modulating role in eating behavior, energy metabolism, and food-related reward processing [Reference Solinas, Goldberg and Piomelli17–Reference D’Addario, Micioni Di Bonaventura, Pucci, Romano, Gaetani and Ciccocioppo20]. This system is composed of endogenous ligands (i.e., endocannabinoids, eCBs), cannabinoid receptors (CBRs), and the enzymatic machinery in charge of the synthesis and degradation of the eCBs [Reference D’Addario, Micioni Di Bonaventura, Pucci, Romano, Gaetani and Ciccocioppo20]. In addition to being the two best-known eCBs [Reference Hanuš, Gopher, Almog and Mechoulam21], anandamide (AEA) and 2-arachidonoylglycerol (2-AG) are involved in homeostatic and hedonic aspects of feeding by pleiotropic actions [Reference Bermudez-Silva, Cardinal and Cota22, Reference Gatta-Cherifi and Cota23], mostly through their union with the type-1 CBR (CB1R) [Reference Pagotto, Marsicano, Cota, Lutz and Pasquali24]. While AEA has a higher affinity than 2-AG, acting as a partial agonist, 2-AG is considered a full CB1R agonist [Reference Di Marzo and De Petrocellis25]. This receptor is predominantly located in the central nervous system (CNS) [Reference DiPatrizio and Simansky19, Reference Piomelli26], where brain 2-AG concentrations are almost 200 times higher than those of AEA [Reference Nomura, Hudak, Ward, Burston, Issa and Fisher27], but also found peripherally (e.g., adipose tissue, gastrointestinal tract, liver, pancreas, skeletal muscle, and kidney) [Reference Pagotto, Marsicano, Cota, Lutz and Pasquali24, Reference Matias and Di Marzo28].
Globally, the eCB system exerts a central orexigenic function [Reference Cota, Marsicano, Tschöp, Grübler, Flachskamm and Schubert29] as a retrograde inhibitor of dopaminergic neurotransmission in both regulatory pathways of intake, homeostatic and hedonic [Reference Gatta-Cherifi30, Reference Coccurello and Maccarrone31]. As part of the homeostatic mechanism, the eCB system is involved in the integration of peripheral and central hunger and satiety signals in the hypothalamus, promoting behaviors toward food acquisition [Reference D’Addario, Micioni Di Bonaventura, Pucci, Romano, Gaetani and Ciccocioppo20, Reference Berridge and Robinson32]. In the hedonic pathway, the eCB system modulates mesolimbic circuits that are involved in increasing motivation toward food (i.e., “wanting to eat” psychological process) and reinforcing the rewarding properties of food (i.e., “liking eating” psychological process) [Reference Berridge and Robinson32, Reference Jager and Witkamp33]. AEA has been classically defined as a physiological meal initiator, increasing motivation toward food (i.e., “wanting to eat”) [Reference Gatta-Cherifi30] and the hedonic aspects of food (i.e., “liking eating”) [Reference Berridge34, Reference Mahler, Smith and Berridge35]. 2-AG has been mostly related to reinforcing the rewarding properties of food (i.e., “liking eating”) [Reference Monteleone, Di Marzo, Monteleone, Dalle Grave, Aveta and El Ghoch36], suggesting a distinctive role to each one [Reference Monteleone, Di Marzo, Monteleone, Dalle Grave, Aveta and El Ghoch36, Reference Mazier, Saucisse, Gatta-Cherifi and Cota37]. In addition, the eCB system promotes peripherally anabolic processes toward energy storage [Reference Matias, Gonthier, Orlando, Martiadis, De Petrocellis and Cervino38], with increased concentrations during fasting and decreased after feeding at both CNS and peripheral tissues [Reference Kirkham, Williams, Fezza and Di Marzo39]. A bidirectional cannabinoid signaling between brain regions and peripheral tissues has been described, which might contribute to intrinsically regulating the activity of the eCB system [Reference Mazier, Saucisse, Gatta-Cherifi and Cota37, Reference Berland, Castel, Terrasi, Montalban, Foppen and Martin40–Reference Yagin, Aliasgari, Alizadeh, Aliasgharzadeh and Mahdavi45]. Interestingly, in recent years, the gut–brain vagal axis has received special attention due to its potential role in regulating energy balance [Reference Clemmensen, Müller, Woods, Berthoud, Seeley and Tschöp46]. This axis seems to modulate central homeostatic and hedonic feeding pathways through the signaling of several peripheral endocrine factors (e.g., ghrelin, leptin, etc.), including peripheral eCBs [Reference Berland, Castel, Terrasi, Montalban, Foppen and Martin40].
In the context of EWC, studies have hypothesized alterations in the eCB system could underlie maladaptive behavior [Reference Hirsch and Tam47]. In obesity, a hyperactive eCB system has been described, with increased CB1R availability [Reference Osei-Hyiaman, DePetrillo, Pacher, Liu, Radaeva and Bátkai48], as well as AEA and/or 2-AG concentrations during fasting [Reference Matias, Gonthier, Orlando, Martiadis, De Petrocellis and Cervino38, Reference Pastor, Fernández-Aranda, Fit, Jiménez-Murcia, Botella and Fernández-Real49–Reference Engeli51]. In patients with BED, higher AEA concentrations have been observed compared with healthy controls (HCs), hypothesizing that increased AEA in BED could be a risk factor for BEs [Reference Monteleone, Matias, Martiadis, De Petrocellis, Maj and Di Marzo52]. However, in patients with BN, who also report BE, no significant differences have been found compared with HC [Reference Monteleone, Matias, Martiadis, De Petrocellis, Maj and Di Marzo52]. Although a hypoactive eCB system has been stated in AN [Reference Di Marzo, Ligresti and Cristino53–Reference Gérard, Pieters, Goffin, Bormans and Van Laere55], describing a lower CB1R availability [Reference Collu, Scherma, Piscitelli, Giunti, Satta and Castelli56], findings related to the amount of eCBs remain still inconclusive [Reference Monteleone, Matias, Martiadis, De Petrocellis, Maj and Di Marzo52, Reference Piccolo, Claussen, Bluemel, Schumacher, Cronin and Fried57]. Monteleone et al. [Reference Monteleone, Matias, Martiadis, De Petrocellis, Maj and Di Marzo52] described higher plasma AEA concentrations in AN compared with HC, whereas a recent study reported lower AEA concentrations in acute phases of the disorder and post-recovery [Reference Piccolo, Claussen, Bluemel, Schumacher, Cronin and Fried57], which was also supported in animal models [Reference Collu, Scherma, Piscitelli, Giunti, Satta and Castelli56]. Regarding 2-AG, studies in EDs have shown no significant differences in 2-AG concentrations when comparing BSD or AN with HC [Reference Yagin, Aliasgari, Alizadeh, Aliasgharzadeh and Mahdavi45, Reference Monteleone, Matias, Martiadis, De Petrocellis, Maj and Di Marzo52, Reference Piccolo, Claussen, Bluemel, Schumacher, Cronin and Fried57].
The association between eCBs and body composition has been explored with the intent to provide further insight into the potential underlying neurobiological mechanisms among EWC [Reference Ceccarini, Weltens, Ly, Tack, Van Oudenhove and Van Laere58, Reference Pastor, Farré, Fitó, Fernandez-Aranda and De La Torre59]. In this line, a study in individuals with EDs and obesity described a negative association between BMI and CB1R availability in both hypothalamic (i.e., homeostatic pathway) and mesolimbic regions (i.e., hedonic pathway), supporting the existence of compensatory mechanisms that seek to counteract the abnormal activity of the eCB system in EWC [Reference Ceccarini, Weltens, Ly, Tack, Van Oudenhove and Van Laere58]. On the other hand, in HC, CB1R availability was inversely linked to BMI, but only regarding the homeostatic pathway [Reference Ceccarini, Weltens, Ly, Tack, Van Oudenhove and Van Laere58]. In the general population, a study exploring a wide range of BMI observed higher 2-AG concentrations in subjects with obesity and lower AEA in individuals with underweight [Reference Pastor, Fernández-Aranda, Fit, Jiménez-Murcia, Botella and Fernández-Real49]. These interactions between the eCB system and anthropometric measurements such as BMI might preliminarily indicate the existence of different functional links among individuals with different body compositions.
From a psychological perspective, the eCB system has shown to be involved in the pathogenesis of mood disturbances and impulse control problems [Reference Solinas, Yasar and Goldberg60–Reference Fitzgerald, Chesney, Lee, Brasel, Larson and Hillard67]. Indeed, the role of eCBs has been explored in some psychiatric disorders such as addictive-related disorders [Reference Solinas, Yasar and Goldberg60, Reference Laksmidewi and Soejitno61], borderline personality disorder [Reference Wingenfeld, Dettenborn, Kirschbaum, Gao, Otte and Roepke62, Reference Schaefer, Enning, Mueller, Bumb, Rohleder and Odorfer63], posttraumatic stress disorder (PTSD) [Reference Hill, Campolongo, Yehuda and Patel64–Reference Sloan, Grant, Gowin, Ramchandani and Le Foll66], and depression [Reference Fitzgerald, Chesney, Lee, Brasel, Larson and Hillard67]. However, findings are mixed so far. For instance, while studies have described an elevated availability of CB1R in the brain of patients with PTSD [Reference Neumeister, Normandin, Pietrzak, Piomelli, Zheng and Gujarro-Anton65], other studies have shown elevated [Reference Schaefer, Enning, Mueller, Bumb, Rohleder and Odorfer63] or reduced [Reference Wingenfeld, Dettenborn, Kirschbaum, Gao, Otte and Roepke62, Reference Kolla, Mizrahi, Karas, Wang, Bagby and McMain68] circulating eCBs concentrations. In the context of EWC, the evidence exploring the clinical interactions of eCBs is scarce. Preliminary results have reported an association between CB1R down-regulation and EDs severity and personality traits such as novelty-seeking and perfectionism [Reference Schroeder, Eberlein, de Zwaan, Kornhuber, Bleich and Frieling69, Reference Frieling, Albrecht, Jedtberg, Gozner, Lenz and Wilhelm70], suggesting a potential role in the psychopathology of EWC.
In this line, some investigations have explored the eCB system as a potential pharmacological target for treating mood-related disorders [Reference Blankman and Cravatt71] and obesity with BE [Reference Pataky, Gasteyger, Ziegler, Rissanen, Hanotin and Golay72]. While studies have suggested that increased AEA concentrations might have an antidepressant and anxiolytic effect in both animal and human models [Reference de Morais, de Souza, da Silva, Ferreira, Baggio and Vanvossen73–Reference Støving, Andries, Brixen, Flyvbjerg, Hørder and Frystyk75], in obesity with BE, the CB1R blockade has shown effects in reducing food intake and, even, weight and adiposity [Reference Hirsch and Tam47, Reference Reas and Grilo76]. However, clinical trials have not been successful given the side effects of pharmacological treatments [Reference Blankman and Cravatt71, Reference Sam, Salem and Ghatei77]. To date, the evidence obtained requires further studies to consolidate these findings. The peripheral eCB system should be considered as a potential therapeutic target [Reference Ruiz de Azua and Lutz78], supported by the existence of bottom-up cannabinoid signaling (e.g., gut–brain axis) [Reference Berland, Castel, Terrasi, Montalban, Foppen and Martin40, Reference Bellocchio, Soria-Gómez, Quarta, Metna-Laurent, Cardinal and Binder41, Reference DiPatrizio, Astarita, Schwartz, Li and Piomelli43] potentially involved in the pathophysiology of EWC [Reference Berland, Castel, Terrasi, Montalban, Foppen and Martin40, Reference Izzo, Piscitelli, Capasso, Aviello, Romano and Borrelli44] and opening the possibility of minimizing side effects [Reference Hirsch and Tam47].
Given this background, our initial objective was to evaluate differences in fasting circulating AEA and 2-AG concentrations in individuals with EWC compared with HC. Furthermore, aiming to explore the interaction between circulating AEA and 2-AG concentrations, BMI, and clinical variables, we investigated the underlying role of eCBs in each clinical group. We hypothesized obesity groups without (OB-ED) and with ED (OB+ED) would exhibit increased eCBs concentrations compared with the AN and HC group while the AN group would report the lowest eCBs concentrations. Considering the distinctive role of both 2-AG and AEA on food intake, we expected characteristic associations with BMI and clinical variables in each clinical group.
Methods
Participants
A total of 113 adult women (18–56 years old) were recruited: 57 individuals had obesity, 37 OB-ED and 20 OB+ED (3 BN and 17 BED); and 27 individuals had AN (25 restrictive and 2 binge-purging subtypes). Clinical groups were compared with 29 HC (BMI = 18–24.99 kg/m2). Individuals with EDs were diagnosed according to DSM-5 criteria [5], using a semi-structured interview based on the SCID-5 [Reference First, Williams, Karg and Spitzer79]. Participants from the AN and OB+ED group were recruited from the Eating Disorders Unit at the Bellvitge University Hospital (Barcelona, Spain), while those individuals with OB-ED were recruited from the Endocrinology and Nutrition Unit at the same hospital. The HC group was recruited via advertisements from the same catchment area. In those with EDs, inclusion in the study occurred within the first week of treatment admission.
All participants underwent the Mini-International Neuropsychiatric Interview (M.I.N.I.) [Reference Ferrando, Bobes, Gibert, Soto and Soto80] to assess the presence of a psychiatric disorder. In the case of HC, exclusion criteria were a lifetime history of ED, based on DSM-5 diagnostic criteria, and/or obesity, and a current diagnosis of a psychiatric disorder. The study deferral criteria for all participants were male sex, the presence of an organic mental disorder, or an intellectual disability, as well as current problematic use of alcohol and illicit drugs (e.g., cannabis or cocaine).
Procedures
Participants were evaluated at Eating Disorders Unit (Bellvitge University Hospital, Barcelona, Spain) by experienced clinical psychologists and psychiatrists in two separate sessions. The first session consisted of a semi-structured clinical interview and self-report questionnaires that are part of the standardized psychometric assessment routinely performed in the initial clinical evaluation in our treatment unit. These psychometric instruments are designed at assessing general psychopathology, emotion regulation, and impulsivity. The second session consisted of measuring BMI and collecting fasting blood samples to assess circulating AEA and 2-AG concentrations.
Ethics
The study was carried out according to Good Clinical Practice and the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of the Bellvitge University Hospital (PR146/14). All participants were thoroughly informed of the procedures and provided written informed consent.
Assessments
Anthropometric measures
Height was measured by a stadiometer without wearing shoes. This information was introduced in a leg-to-leg body composition analyzer using a Tanita BC-420MA (Tanita BC-420MA, Tanita Corp., Tokyo, Japan) to collect body composition variables and obtain BMI. This instrument is a noninvasive bioelectrical impedance analyzer that estimates body composition, considering age and sex.
Biological measures
Blood samples were obtained in the morning, after at least 12 hours of fasting. Blood was processed at 1,700 g in a refrigerated centrifuge (4°C) over 20 min. Plasma was separated immediately and stored at −80°C until its analysis. AEA and 2-AG were analyzed by liquid chromatography-mass spectrometry (LC/MS–MS) with a previously validated method [Reference Pastor, Farré, Fitó, Fernandez-Aranda and De La Torre59].
Clinical measures
Symptom Checklist-90 Items-Revised (SCL-90-R) [Reference Derogatis81]; Spanish validation [Reference Derogatis82]. The SCL-90-R assesses nine scales on general psychopathology: somatization, obsessive–compulsive, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, and psychoticism. In addition, it assesses three global psychological distress indices: Global Severity Index (GSI), Positive Symptom Total (PST), and Positive Symptom Distress Index (PSDI). The internal consistency in our sample was α = 0.98.
Yale Food Addiction Scale 2.0 (YFAS 2.0) [Reference Gearhardt, Corbin and Brownell83]; Spanish validation [Reference Granero, Jiménez-Murcia, Gerhardt, Agüera, Aymamí and Gómez-Peña84]. This is a self-reported scale to assess food addiction (FA) based on the 11-substance dependence-related symptoms adapted to the context of food consumption. The YFAS 2.0 consists of 35 items and produces two measurements: (a) a continuous symptom count score that reflects the number of fulfilled diagnostic criteria (ranging from 0 to 11), and (b) a binary measurement (present versus absent) based on the number of symptoms (at least 2) and the self-reported clinically impairment or distress. Additionally, it gives the severity cut-offs: mild (2–3 symptoms), moderate (4–5 symptoms), and severe (6–11 symptoms). The internal consistency of our sample was α = 0.97.
Difficulties in Emotion Regulation Strategies (DERS) [Reference Gratz and Roemer85]; Spanish validation [Reference Wolz, Agüera, Granero, Jiménez-Murcia, Gratz and Menchón86]. This is a 36-item self-reported scale to assess emotional dysregulation, divided into six subscales: lack of emotional awareness, lack of emotional clarity, nonacceptance of emotional responses, difficulties engaging in goal-directed behavior, limited access to emotional regulation strategies, and impulse control difficulties. Participants responded using a five-point Likert scale ranging from 1 (rarely) to 5 (almost always). Higher scores indicate greater problems in emotion regulation. The internal consistency of the DERS total score in our sample was α = 0.96.
Impulsive Behavior Scale (UPPS-P) [Reference Whiteside, Lynam, Miller and Reynolds87]; Spanish validation [Reference Verdejo-García, Lozano, Moya, Alcázar and Pérez-García88]. It measures five facets of impulsive behavior through self-report on 59 items: negative urgency; positive urgency; lack of premeditation; lack of perseverance; and sensation-seeking. The internal consistency in this study was α = 0.90.
Statistical analysis
Statistical analysis was carried out with Stata17 for Windows [89]. Comparisons between groups were done with chi-square tests (χ2) for categorical variables, and analysis of variance (ANOVA) for quantitative variables. A statistical power calculation was previously performed for the mean comparisons displaying values ranging from 1 − β = 0.81 to 1 − β = 0.89, a threshold usually considered acceptable in medical science (1 − β = 0.80). Differences between groups in 2-AG and AEA concentrations were done with an analysis of covariance (ANCOVA), adjusted by the participants’ age. Fisher’s least significant difference method was employed for multiple comparisons, and standardized Cohen’s-d statistic assessed the effect size of the mean differences (low–poor effect size was interpreted for |d| > 0.20, moderate–medium for |d| > 0.50, and large–high for |d| > 0.80) [Reference Cohen90].
Finally, a path analysis procedure was conducted to explore underlying relationships between biological variables and clinical features. This statistical procedure is an extension of multiple regression modeling and estimates the magnitude and significance of a set of relationships between variables, including mediational links (direct and indirect effects) [Reference Kline91]. Path analysis was run as a case of structural equation modeling (SEM) with the maximum-likelihood estimation method. To assess the invariance of the structural coefficients between the diagnostic types a multi-group model was defined and tested. Goodness-of-fit was evaluated using standard statistical measures and adequate fitting was considered for: nonsignificant result for the χ2 test, the root mean square error of approximation (RMSEA) < 0.08, Bentler’s Comparative Fit Index (CFI) > 0.90, Tucker–Lewis Index (TLI) > 0.90, and the standardized root mean square residual (SRMR) < 0.10 [Reference Barrett92]. The coefficient of determination (CD) measured the global predictive capacity of the model. In this study, SEM was obtained for each clinical group.
Results
Descriptive of the sample
Table 1 displays the distribution of the socio-demographic, BMI, and clinical variables (total scores), and the comparison between groups. As expected, significant differences between groups were found in BMI (p < 0.001*), UPPS-P (p = 0.001*), SCL-90R GSI (p < 0.001*), DERS (p < 0.001*), Y-FAS 2.0 (p < 0.001*), but also in age (p < 0.001*). For this reason, age was considered confounding.
Table 1. Descriptive of the sample

Abbreviations: AN, anorexia nervosa; DERS, Difficulties in Emotion Regulation Scale; HCs, healthy controls; OB+ED, obesity with eating disorder; OB-ED, obesity without eating disorder; SCL-90-R GSI, Symptom Checklist-90-Revised, global severity index; SD, standard deviation; UPPS-P, Impulsive Behavior Scale; YFAS-2, Yale Food Addiction Scale.
* Bold: Significant comparison (0.05 level).
Comparison of biological measures between the groups
Table 2 displays the results of the ANCOVA (adjusted by age), comparing 2-AG and AEA between groups. Regarding 2-AG, differences between HC and obesity groups (i.e., OB-ED and OB+ED) were observed, displaying the HC group with significantly higher mean concentrations. On the other hand, obesity groups registered the highest AEA mean concentrations (0.45 and 0.38, respectively), which statistically differed from those registered in the AN and HC groups (0.22 and 0.25, respectively). Figure 1 shows the scatterplots displaying the relationships between 2-AG and AEA with BMI. The plots evidence the moderator role of the ED subtype: (a) for 2-AG a negative relationship was identified with BMI among HC and AN, while no significant association was found between OB+ED and OB-ED group; and (b) for AEA, a positive association was found with BMI among OB-ED, a negative association among AN, and a nonsignificant association was identified among HC and OB+ED conditions.
Table 2. Comparison between groups: ANCOVA adjusted by age
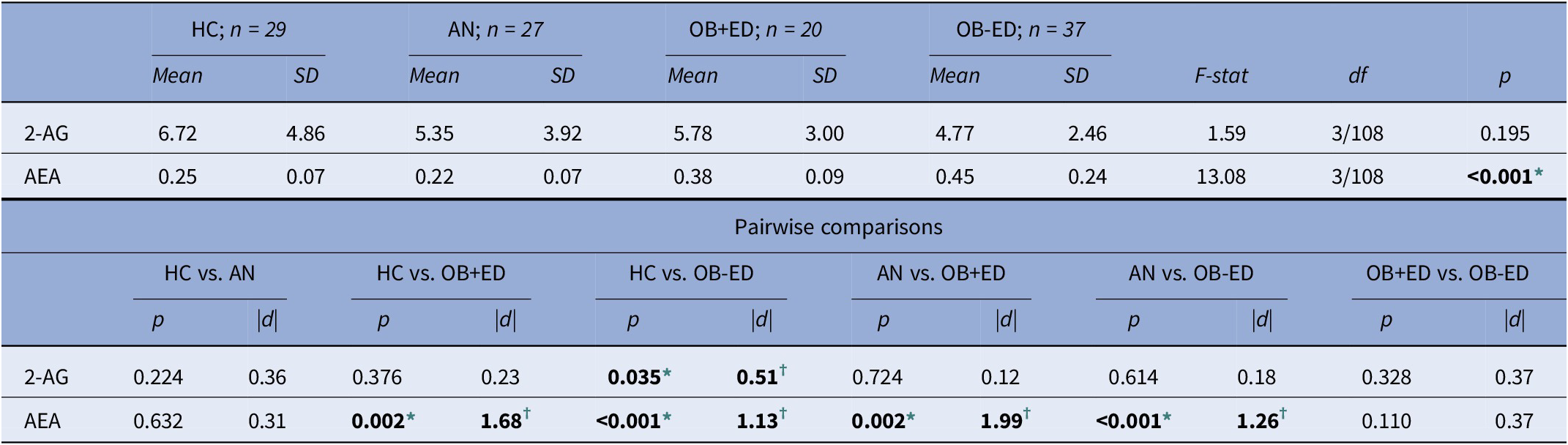
Abbreviations: 2-AG, 2-arachidonoylglycerol; AEA, anandamide; AN, anorexia nervosa; HCs, healthy controls; OB+ED, obesity with eating disorder; OB-ED, obesity without eating disorder; SD, standard deviation.
* Bold: Significant comparison (0.05 level).
† Bold: Effect size into ranges mild–moderate (|d| > 0.50) to high–large (|d| > 0.80).

Figure 1. The scatterplot displays regression analysis of the association of circulating 2-arachidonoylglycerol (2-AG) and anandamide (AEA) concentrations with body mass index (BMI). Continuous line: regression line. Green line: regression line in healthy controls. Red line: regression line in anorexia nervosa. Blue line: regression line in obesity with eating disorder. Black line: regression line in obesity without eating disorder. 2-AG, 2-arachidonoylglycerol (ng/ml); AEA, anandamide (ng/ml); BMI, body mass index (kg/m2); HCs, healthy controls; AN, anorexia nervosa; OB+ED, obesity with eating disorder; OB-ED, obesity without eating disorder.
Path analysis
The multi-group model assessing the invariance by the diagnostic types achieved adequate fitting: χ2 = 16.06 (p = 0.378), RMSEA = 0.050, CFI = 0.991, TLI = 0.948, and SRMR = 0.090. The global predictive capacity of the model was CD = 0.187. The joint test for invariance obtained significant results (χ2 = 72.35, p = 0.001), indicating that the set of relationships between variables was different among diagnostic groups.
Figure 2 shows the path diagram with standardized coefficients for each clinical group. To facilitate interpretation, only significant relationships have been plotted (nonsignificant parameters have been deleted in the figure). Coefficients with statistical differences between groups are represented in red lines whereas black lines represent no statistical differences. Multi-group SEM for the complete sample can be viewed in the Supplementary Material (Figure S1).

Figure 2. Path diagram: standardized coefficients (results adjusted by age) (in color). Continuous line: significant parameter. Dash line: nonsignificant parameter. Black line: invariant parameter (the coefficient is statistically equal between the diagnostic subtypes). Red line: noninvariant parameter (the coefficient is statistically different between the diagnostic subtypes). 2-AG, 2-arachidonoylglycerol; AEA, anandamide; AN, anorexia nervosa; BMI, body mass index; DERS, Difficulties in Emotion Regulation Scale; OB+ED, obesity with eating disorder; OB-ED, obesity without eating disorder; SCL-90-R GSI, Symptom Checklist-90-Revised, global severity index; UPPS-P, Impulsive Behavior Scale; YFAS-2, Yale Food Addiction Scale.
In the AN group, higher 2-AG concentrations predicted a worse psychopathological state, while lower AEA concentrations predicted higher BMI and higher emotional dysregulation levels (DERS). The UPPS-P and YFAS scores were also higher for patients with higher emotional dysregulation levels, while the BMI was also higher in patients with higher DERS scores but lower general psychopathology.
In the OB+ED group, higher AEA concentrations contributed to increasing YFAS scores and BMI and decreased DERS total score. UPPS-P and YFAS were also increased for patients with higher emotional dysregulation levels. Higher impulsivity levels were related to a worse psychopathological state. In the model, no significant associations were observed for 2-AG with other variables.
In the OB-ED group, higher AEA concentrations predicted higher YFAS scores and BMI, and lower DERS total scores. UPPS-P was increased for patients with higher emotional dysregulation scores, while YFAS total score was higher for patients with a worse psychopathological state. In the model, no significant associations were observed for 2-AG with other variables.
A common mediational link was observed between AEA concentrations and specific clinical features within the three diagnostic groups: lower AEA concentrations predicted a higher DERS total score, which, in turn, predicted a worse psychopathological state.
Discussion
The present study found higher AEA concentrations in the obesity groups compared with the HC and AN group, as well as higher 2-AG concentrations in the HC group compared with the OB-ED group. Interestingly, AEA concentrations showed a distinct association with BMI among EWC. In AN, higher AEA concentrations predicted lower BMI, whereas, in the obesity groups, increased AEA concentrations were linked to higher BMI and FA. In all clinical groups, higher AEA concentrations were related to lower emotional dysregulation and indirectly predicted lower general psychopathology. Emotional dysregulation also mediated the relationship between AEA and impulsivity. Higher 2-AG concentrations predicted greater general psychopathology in the AN group.
Differences between groups in circulating eCBs concentrations partially supported our hypotheses. On the one hand, the obesity groups (i.e., OB-ED and OB+ED) exhibited similar AEA concentrations, which were significantly higher than in the HC and AN group, respectively. We expected to obtain elevated circulating eCBs concentrations in individuals with obesity, according to previous studies [Reference Matias, Gonthier, Orlando, Martiadis, De Petrocellis and Cervino38, Reference Pastor, Fernández-Aranda, Fit, Jiménez-Murcia, Botella and Fernández-Real49, Reference Côté, Matias, Lemieux, Petrosino, Alméras and Després50, Reference Monteleone, Matias, Martiadis, De Petrocellis, Maj and Di Marzo52]. Increased AEA concentration supported the rationale that AEA could be a vulnerability factor for overeating in obesity and BSD [Reference D’Addario, Micioni Di Bonaventura, Pucci, Romano, Gaetani and Ciccocioppo20, Reference Monteleone, Matias, Martiadis, De Petrocellis, Maj and Di Marzo52, Reference Gearhardt, Yokum, Orr, Stice, Corbin and Brownell93], as well as a risk factor for the onset and maintenance of BE [Reference Monteleone, Matias, Martiadis, De Petrocellis, Maj and Di Marzo52, Reference Monteleone94]. Considering peripheral eCBs also influence vagal-dependent activity at the central level, our results raise the question of whether AEA specifically may play a key role in the pathophysiology of obesity and BED through the gut–brain vagal axis, underlying BE by triggering both homeostatic and hedonic brain circuits [Reference Berland, Castel, Terrasi, Montalban, Foppen and Martin40]. Besides, due to the modulation of eCBs on other endocrine processes in peripheral tissues such as the gastrointestinal tract and liver [Reference Monteleone and Maj42, Reference Yagin, Aliasgari, Alizadeh, Aliasgharzadeh and Mahdavi45, Reference Osei-Hyiaman, DePetrillo, Pacher, Liu, Radaeva and Bátkai48], this increase of AEA would also underlie other metabolic disorders, which are highly comorbid in obesity (with and without EDs) (e.g., diabetes mellitus, dyslipidemia, etc.) [Reference Giel, Bulik, Fernandez-Aranda, Hay, Keski-Rahkonen and Schag10, Reference Matias, Gonthier, Orlando, Martiadis, De Petrocellis and Cervino38].
On the other hand, only the OB-ED group significantly differs in 2-AG concentrations from the HC group, surprisingly showing lower 2-AG concentrations. Considering this unexpected result, we emphasize the need for experimental research to explore the different factors, physiological pathways, and biofeedback mechanisms that seem to be involved in promoting the eCB system actions [Reference Mazier, Saucisse, Gatta-Cherifi and Cota37, Reference Berland, Castel, Terrasi, Montalban, Foppen and Martin40, Reference Izzo, Piscitelli, Capasso, Aviello, Romano and Borrelli44, Reference Monteleone, Tortorella, Martiadis, Di Filippo, Canestrelli and Maj95, Reference Pucci, Micioni Di Bonaventura, Zaplatic, Bellia, Maccarrone and Cifani96]. In this line, genetic alterations modifying the enzymatic activity of the eCB system could be involved in a dysfunctional eCBs synthesis [Reference Monteleone, Tortorella, Martiadis, Di Filippo, Canestrelli and Maj95–Reference Sipe, Waalen, Gerber and Beutler98]. For example, fatty acid amide hydrolase (FAAH) gene polymorphisms have been described in obesity [Reference Monteleone, Tortorella, Martiadis, Di Filippo, Canestrelli and Maj95, Reference Sipe, Waalen, Gerber and Beutler98], an enzyme aimed at eCBs degradation, specially AEA [Reference Ruiz de Azua and Lutz78, Reference McKinney and Cravatt99]. Other animal and human works have described increased eCBs concentrations linked to a reduced central and peripheral expression of FAAH in obesity [Reference Monteleone, Tortorella, Martiadis, Di Filippo, Canestrelli and Maj95, Reference Sipe, Waalen, Gerber and Beutler98], being this enzyme even proposed as a potential biomarker of BE [Reference Pucci, Micioni Di Bonaventura, Zaplatic, Bellia, Maccarrone and Cifani96]. Although our study did not further investigate genetic factors underlying eCBs concentrations, future studies should assess whether specific genetic polymorphisms such as those related to FAAH or more specific enzymes responsible for the metabolism of 2-AG [Reference Hirsch and Tam47] would underlie differences in circulating eCBs.
In the AN group, the lack of differences in 2-AG concentrations when compared with HC were in line with previous studies [Reference Monteleone, Matias, Martiadis, De Petrocellis, Maj and Di Marzo52, Reference Piccolo, Claussen, Bluemel, Schumacher, Cronin and Fried57]. Regarding AEA, this group showed significantly lower AEA concentrations compared with the obesity groups, although these differences were not observed between the AN and HC group. Despite this latter finding contrasted with previous works [Reference Monteleone, Matias, Martiadis, De Petrocellis, Maj and Di Marzo52, Reference Piccolo, Claussen, Bluemel, Schumacher, Cronin and Fried57], this lack of differences could respond to a compensatory mechanism secondary to a hypoactive eCB system in AN [Reference Di Marzo, Ligresti and Cristino53–Reference Gérard, Pieters, Goffin, Bormans and Van Laere55]. As speculative, this fact could be understood as an intent of the eCB system to promote food intake in AN through the stimulation of the homeostatic pathway [Reference Casteels, Gérard, Van Kuyck, Pottel, Nuttin and Bormans54, Reference Gérard, Pieters, Goffin, Bormans and Van Laere55, Reference Schroeder, Eberlein, de Zwaan, Kornhuber, Bleich and Frieling69]. In addition, considering the role of AEA in motivational reward processing [Reference Berridge and Robinson32, Reference Jager and Witkamp33], a plausible hypothesis addressed by Monteleone [Reference Monteleone94] would suggest that increased AEA concentrations may also act by reinforcing self-starvation which would allow patients with AN to cope with the sensation of hunger despite prolonged restriction [Reference Monteleone94]. Whether this hypothesis [Reference Monteleone94] may help to explain our result, the cross-sectional nature of our study did not allow us to confirm it. However, the association between AEA and BMI in the clinical groups could preliminarily support this rationale.
Noticeably, the SEM analysis showed that higher AEA predicted lower BMI in AN whereas higher BMI in the obesity groups, suggesting that an elevated AEA might be a potential indicator of a more extreme BMI in each clinical condition. Besides, as we expected, AEA and 2-AG showed different links with BMI and psychological variables in the clinical groups. For instance, 2-AG was only related to BMI in the AN group. In this case, similar to a previous study [Reference La Porta, Andreea Bura, Llorente-Onaindia, Pastor, Navarrete and García-Gutierrez74], higher 2-AG concentrations were related to greater general psychopathology, which acted as a mediational factor in predicting a lower BMI. This result is interesting because previous studies have pointed to the association of general psychopathology (e.g., anxiety, depressive symptoms, hostility, etc.) with lower BMI and greater severity in AN [Reference Toppino, Longo, Martini, Abbate-Daga and Marzola100, Reference Forrest, Jones, Ortiz and Smith101]. Then, our SEM analysis may delineate the existence of a potential endophenotype characterized by the interplay between 2-AG and general psychopathology that would particularly predict BMI in AN, a criterion of severity in this disorder [102].
Interestingly, all clinical groups showed a common pathway related to AEA and some clinical factors. Thus, lower AEA predicted higher emotional dysregulation, which also mediated greater general psychopathology. In addition, a higher emotional dysregulation predicted greater impulsivity. The clinical associations observed in the SEM analysis were in line with previous literature [Reference Prefit, Cândea and Szentagotai-Tătar103–Reference Benzerouk, Djerada, Bertin, Barrière, Gierski and Kaladjian105], as well as the potential association between these psychological variables and BMI in EWC [Reference Toppino, Longo, Martini, Abbate-Daga and Marzola100, Reference Forrest, Jones, Ortiz and Smith101, Reference Testa, Baenas, Vintró-Alcaraz, Granero, Agüera and Sánchez106]. Moreover, AEA has been linked to emotional processing and impulsivity in other psychiatric disorders [Reference Solinas, Yasar and Goldberg60–Reference Fitzgerald, Chesney, Lee, Brasel, Larson and Hillard67, Reference Gunduz-Cinar, MacPherson, Cinar, Gamble-George, Sugden and Williams107, Reference Gärtner, Dörfel, Diers, Witt, Strobel and Brocke108] being, for example, lower AEA concentrations linked to higher emotion dysregulation [Reference Neumeister, Normandin, Pietrzak, Piomelli, Zheng and Gujarro-Anton65, Reference Kolla, Mizrahi, Karas, Wang, Bagby and McMain68]. In EWC, these results pointed to the possible participation of peripheral eCBs along with psychological processes involved in impulsivity, emotional regulation, and mood that may modulate feeding behavior [Reference Prefit, Cândea and Szentagotai-Tătar103–Reference Benzerouk, Djerada, Bertin, Barrière, Gierski and Kaladjian105]. Besides, higher AEA concentrations predicted higher FA, which is highly prevalent in individuals with obesity [Reference Granero, Jiménez-Murcia, Gerhardt, Agüera, Aymamí and Gómez-Peña84, Reference Gearhardt, White, Masheb, Morgan, Crosby and Grilo109–Reference Meule, Von Rezori and Blechert113]. Particularly, the association between AEA and FA was also mediated by emotional dysregulation in the OB+ED group and by general psychopathology in the OB-ED group. In individuals with BSD, the co-occurrence of ED and FA has been associated with greater emotional dysregulation and general psychopathology compared with those without FA [Reference Gearhardt, White, Masheb, Morgan, Crosby and Grilo109]. In obesity, the presence of FA has been related to depressive symptoms and impulsivity traits [Reference Davis, Curtis, Levitan, Carter, Kaplan and Kennedy114]. The specific clinical pathways of AEA could imply a differential pattern characterizing individuals with obesity with or without a diagnosis of ED. However, higher FA scores in OB-ED could draw a clinical profile more similar to OB+ED. These findings reinforce the notion that AEA may represent a shared vulnerability factor underlying transdiagnostic psychological features not only among different psychiatric disorders including EDs [Reference Vintró-Alcaraz, Munguía, Granero, Gaspar-Pérez, Solé-Morata and Sánchez115, Reference Munguía, Jiménez-Murcia, Granero, Baenas, Agüera and Sánchez116], but also in OB-ED in the context of EWC.
Altogether, the eCB system could be a crucial pharmacological target regarding its involvement in the regulation of food intake and weight management [Reference Sam, Salem and Ghatei77, Reference Pataky, Gasteyger, Ziegler, Rissanen, Hanotin and Golay117–Reference Pi-Sunyer, Aronne, Heshmati, Devin and Rosenstock119], as well as in psychopathological processes among individuals with EWC. Consistent with this notion, the eCB system has been proposed as a therapeutic target in EWC to manage the metabolic comorbidities and cardiovascular risk factors linked, for example, to obesity such as dyslipidemia and diabetes mellitus [Reference Pi-Sunyer, Aronne, Heshmati, Devin and Rosenstock119, Reference Van Gaal, Rissanen, Scheen, Ziegler and Rössner120]. Likewise, it has been postulated effective drugs to treat endocrine-related diseases, which contemplate interrelations between the eCB system and other endocrine pathways (e.g., thyroid hormones, estrogens, glucose metabolism, etc.), might be potential candidates to essay among EWC [Reference Borowska, Czarnywojtek, Sawicka-Gutaj, Woliński, Płazińska and Mikołajczak121].
The present study should be considered in light of some limitations, such as a sample consisting of women seeking treatment. Therefore, it does not represent the general population with AN and obesity (with and without ED). Besides, the cross-sectional design did not allow us to infer causality from our results. Moreover, our study did not investigate the effect of purging behaviors (in the OB+ED group), as well as some factors such as sex hormones, medication, or the effect of excessive physical activity (in AN), which would influence the eCB system functioning [Reference Krolick, Zhu and Shi122]. Likewise, although the use of cannabis and other illicit drugs was controlled, tobacco use was not an exclusion criterion. In the future, studies should not disregard its effect on eCBs concentrations. Finally, considering the influence of physiological hunger and satiety signals, further research should analyze circulating eCBs in both fasting and postprandial conditions to accurately report the changes in circulating concentrations of 2AG and AEA and evaluate if there are differences between them. Notwithstanding these limitations, to the best of our knowledge, this is the first study to show the complex interplay between eCBs and psychological variables in EWC. Although several variables could be considered in future studies as confounders, this study did include the use of a previously validated procedure to obtain plasma eCBs concentrations, the presence of a control group, and adjusting for age.
Conclusions
Our results support the notion that AEA and 2-AG have different functional roles in EWC, where AEA predominantly influences BMI and psychological features. In individuals with EDs and obesity, AEA emerges as a possible biological marker of a more extreme BMI and psychopathological profile. In the case of individuals with obesity, although AEA concentrations were similar, the presence or absence of an ED was differentiated by the association of AEA with clinical variables. However, the increase of AEA in OB-ED defined a clinical profile more closely resembling the OB+ED group. Likewise, the interplay between 2-AG and BMI, mediated by general psychopathology, could underlie a more severe profile in individuals with AN. As a result, these findings evidence the implication of eCBs in abnormal eating behavior, weight disturbances, and psychopathological features, which could represent a potential pharmacological target in EWC.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1192/j.eurpsy.2023.2411.
Acknowledgments
We thank CERCA Programme/Generalitat de Catalunya for institutional support. We also thank Instituto de Salud Carlos III (ISCIII), CIBERobn, CIBERSAM and CIBERDEM are initiatives of ISCIII), FEDER funds/European Regional Development Fund (ERDF), a way to build Europe, and the European Social Fund. ESF investing in your future.
Financial support
We thank CERCA Programme / Generalitat de Cataluny a for institutional support. This manuscript and research were supported by grants from the Delegación del Gobierno para el Plan Nacional sobre Drogas (2021I031), Ministerio de Ciencia e Innovación (PDI2021-124887OB-I00), Instituto de Salud Carlos III (ISCIII) (FIS PI20/00132) and cofounded by FEDER funds/European Regional Development Fund (ERDF), a way to build Europe. CIBER Obn, CIBERSAM, and CIBERDEM are initiatives of ISCIII. Additional funding was received by AGAUR- Generalitat de Catalunya (2021-SGR-00824 and 2021 SGR 00253) and European Union’s Horizon 2020 research and innovation program under Grant agreement no. 728018 (H2020-SFS-2016-2, Eat2beNice), no. 847879 (PRIME/H2020, Prevention and Remediation of Insulin Multi morbidity in Europe) and no. 101080219 (eprObes). This study has also been funded by Instituto de Salud Carlos III through the grant CM21/00172 (IB) (Co-funded by European Social Fund. ESF investing in your future). RG is supported by the Catalan Institution for Research and Advanced Studies (ICREA-Academia, 2021-Programme). The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Competing interest
F.F.-A. and S.J.-M. received consultancy honoraria from Novo Nordisk and Fernando Fernández-Aranda editorial honoraria as EIC from Wiley. The remaining authors declared no competing interests.







Comments
No Comments have been published for this article.