1. Introduction
Obsessive-compulsive disorder (OCD) is characterised by intrusive, persistent thoughts that cause distress (obsessions), and/or irrepressible, repetitive behaviour (compulsions). Those cardinal features are seen as a pathology of cognitive control over habits/automated behaviour [Reference Gillan, Morein-Zamir, Urcelay, Sule, Voon and Apergis-Schoute1–Reference Bradbury, Cassin and Rector3]. In line with these behavioural characteristics, neurobiological models hypothesise that OCD is based on a dysfunction of cortical control over basal ganglia circuits, in which automated behaviour is thought to be stored [Reference Graybiel and Rauch4–Reference Baxter6]. Indeed, neuro-imaging functional studies and meta-analyses have mostly found pathological activations in the anterior cingulate cortex (ACC), orbitofrontal cortex (OFC), dorso-lateral prefrontal cortex (DLPFC), and the ventral striatum and thalamus of OCD patients [Reference Rotge, Guehl, Dilharreguy, Cuny, Tignol and Bioulac7–Reference Menzies, Chamberlain, Laird, Thelen, Sahakian and Bullmore9].
These cortical areas, dysfunctional in OCD, send projections to the ventral striatum then back to the cortex via the thalamus, constituting several loops which are involved in associativo-limbic processes such as decision-making, selection of behaviour based on expected reward, and repetitive stereotyped behaviour [1, Reference Burguiere, Monteiro, Mallet, Feng and Graybiel10–Reference Canales and Graybiel12]. The effect of the neuromodulation of the associative and limbic territories of the basal ganglia on the symptoms of patients [Reference Denys, Mantione, Figee, van den Munckhof, Koerselman and Westenberg13–Reference Mallet, Polosan, Jaafari, Baup, Welter and Fontaine15] and animals [Reference Grabli, McCairn, Hirsch, Agid, Feger and Francois16, Reference Worbe, Baup, Grabli, Chaigneau, Mounayar and McCairn17] also supports the crucial role of these loops in the pathophysiology of OCD. An involvement of the motor loop in OCD could be supported by dysfunctional activities found in the supplementary motor area (SMA) or pre-SMA of OCD patients [Reference Page, Rubia, Deeley, Daly, Toal and Mataix-Cols18, Reference Roth, Saykin, Flashman, Pixley, West and Mamourian19], but these results are inconsistent and not confirmed by meta-analyses [Reference Rotge, Guehl, Dilharreguy, Cuny, Tignol and Bioulac7–Reference Menzies, Chamberlain, Laird, Thelen, Sahakian and Bullmore9] nor included in neurocognitive models [Reference Graybiel and Rauch4–Reference Baxter6].
Neuroanatomical studies have demonstrated that each connection within the cortico-basal ganglia loops (e.g. cortico-striatal [Reference Haber, Kim, Mailly and Calzavara20] or pallido-thalamic [Reference Ilinsky, Jouandet and Goldman-Rakic21]) have a functional topography [Reference Haynes and Haber22–Reference McFarland and Haber27]: the medial and ventral part of the loop processes limbic information, the central part associative one, and the lateral and dorsal part processes motor information. This division is supported functionally [Reference Worbe, Baup, Grabli, Chaigneau, Mounayar and McCairn17, Reference Mallet, Schupbach, N'Diaye, Remy, Bardinet and Czernecki28, Reference Karachi, Grabli, Baup, Mounayar, Tande and Francois29]. However, while the organisation is topographic, the anatomo-functional channels are not segregated but overlap partially, possibly allowing an integrated control of behaviour [Reference Haber, Kim, Mailly and Calzavara20, Reference Haynes and Haber22, Reference Mallet, Schupbach, N'Diaye, Remy, Bardinet and Czernecki28, Reference Pidoux, Mahon, Deniau and Charpier30].
We hypothesized that, in OCD, a disturbance in the selection of appropriate behaviour could be linked to a pathological organisation of these anatomo-functional channels. Indeed, most functional connectivity studies using MRI have shown an increased (although some a decreased) correlation of the blood oxygenation levels between cortico-basal ganglia-thalamic nodes in OCD [Reference Rotge, Guehl, Dilharreguy, Cuny, Tignol and Bioulac7–Reference Menzies, Chamberlain, Laird, Thelen, Sahakian and Bullmore9]. However, modifications of the anatomical connections are not known.
While a direct access to theanatomical connectivity of cortico-basal ganglia-thalamic circuits is impossible in humans (it involves ex vivo axonal tracing), probabilistic tractography seems to provide a non-invasive and reliable estimate in vivo [31–Reference Dyrby, Søgaard, Parker, Alexander, Lind and Baaré35]. This method, based on diffusion MRI, follows the orientation of water molecules from one voxel to the next as a proxy for fibre orientation, thus modelling the path of axons through the white matter [Reference Behrens, Woolrich, Jenkinson, Johansen-Berg, Nunes and Clare36, Reference Behrens, Berg, Jbabdi, Rushworth and Woolrich37].
Some studies have already used a diffusion-based approach to white matter with fractional anisotropy (FA). They found modification consistent with the pathophysiological hypotheses of OCD (e.g. in the cingulum which carries fibres to and from the ACC) [Reference Piras, Piras, Caltagirone and Spalletta38–Reference Szeszko, Ardekani, Ashtari, Malhotra, Robinson and Bilder42]. However, FA can only identify punctual modifications of the white matter, and cannot provide information about the state of anatomical connections between regions of interest (ROI), unlike probabilistic tractography [Reference Jones, Knösche and Turner31, Reference Jones43, Reference Schmierer, Wheeler-Kingshott, Boulby, Scaravilli, Altmann and Barker44]. Therefore, our goal was to investigate anatomical connections in OCD using probabilistic tractography.
To our knowledge, this method has not yet been used in OCD. Nevertheless, it has been used to explore anatomical connections in relation to personality traits [Reference Cohen, Schoene-Bake, Elger and Weber45] and neurological disorders of the basal ganglia [Reference Argyelan, Carbon, Niethammer, Uluğ, Voss and Bressman46–Reference Novak, Seunarine, Gibbard, McColgan, Draganski and Friston48]. Those studies were performed at the single voxel level, but animal experiments have shown that the relationship between tractographic metrics and ex-vivo anatomical connectivity is stronger at the macroscopic scale [Reference Gao, Choe, Stepniewska, Li, Avison and Anderson32]. Therefore, we opted for an ROI approach instead of a voxel-by-voxel approach.
Overall, a dysfunction of cortico-basal-ganglia associative and limbic loops in OCD [Reference Graybiel and Rauch4–Reference Baxter6] could be supported anatomically by decreased and/or not properly organised connections. To test this hypothesis, we compared the anatomical connections between limbic and associative cortical (OFC, ACC, DLPFC and the frontal pole) and subcortical (caudate nucleus, putamen and thalamus) ROIs in OCD and healthy controls, using probabilistic tractography. We developed connectivity indices (spatial spread and density) as well as an index of segregation to test 1/a decrease in the strength of connections and 2/a modification of their organisation in OCD compared to controls.
2. Methods
2.1 Participants
We included 37 OCD patients with predominant checking symptoms and 37 healthy controls matched for age and sex. We recruited through a clinical trial (clinicaltrials.gov NCT01331876, Ethics Committee approval 2009-A00652-55) [Reference Morgieve, N'Diaye, Haynes, Granger, Clair and Pelissolo49] and a pathophysiology study (Ethics Committee approval 2007-A00488-45). Only the data at inclusion were used. For both protocols, patients were recruited from outpatient units and through an advertisement on the website of the French OCD association (AFTOC). A clinical psychologist conducted a full interview of each participant and used the Mini International Neuropsychiatric Interview [Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs and Weiller50] as a standardised assessment. Diagnosis of OCD was made according to the Diagnostic and Statistical Manual of mental disorders, 4th edition, revised text (DSM-IV-TR). OCD severity and clinical subtypes were assessed using the Yale-Brown Obsessive-Compulsive Scale (YBOCS), which has an obsession (YBOCS-O) and a compulsion (YBOCS-C) subscale, as well as a checklist which we used to identify the predominant subtype of symptoms (e.g. checking) [Reference Goodman, Price, Rasmussen, Mazure, Fleischmann and Hill51, Reference Mollard, Cottraux and Bouvard52]. Participants completed the Padua Inventory to quantify these subtypes. The inventory comprises four factors, the factor 3 being specific of checking (Padua3) [Reference Sanavio53]. Inclusion criteria for patients were: YBOCS score >16/40, predominant checking symptoms, no axis 1 comorbidity and a stable treatment for at least two months. Controls were free of any axis 1 diagnosis and had no psychotropic medication.
All participants were legal adults and gave a written, informed consent after a complete description of the study by an investigator. The local ethics committee approved all procedures. The authors assert that all procedures contributing to this work comply with the Helsinki Declaration of 1975, as revised in 2008.
2.2 MRI apparatus and procedures
MRI were acquired using a 3T scanner (Siemens TRIO 32 channel TIM) and a 12 channels head coil, including T1 weighted images and diffusion tensor imaging (DTI). Anatomical scans were acquired using axial three-dimensional inversion recovery MP-RAGE (magnetisation-prepared rapid gradient echo) sequences (TR/TE/flip angle: 2.3s/4.18 ms/9°, 208 axial slices, voxel size: 1 × 1 × 1 mm). DTI was performed using echo-planar imaging (TR/TE/flip angle: 12s/86 ms/90°, matrix size: 128 × 128 mm, field of view: 256 × 256 mm, slice thickness: 2 mm, 80 contiguous axial slices, voxel size: 2 × 2 × 2 mm). Diffusion weighting was performed along 50 independent directions, with a b-value of 1000s/mm2, in addition to one reference image (b = 0).
2.3 DTI image processing
Raw DTI images were processed using the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain Software Library (http://www.fmrib.ox.ac.uk/fsl/index.html) [Reference Smith, Jenkinson, Woolrich, Beckmann, Behrens and Johansen-Berg54, Reference Jenkinson, Beckmann, Behrens, Woolrich and Smith55], specifically its Diffusion Toolbox (FDT) [Reference Behrens, Berg, Jbabdi, Rushworth and Woolrich37]. Images were corrected for head motion and eddy currents.
2.4 Segmentation of regions of interest
As written in the Introduction, we aimed to test the anatomical connections within the associative and limbic cortico-basal ganglia circuits in OCD. Based on the results of functional imaging studies and meta-analyses in OCD [Reference Rotge, Guehl, Dilharreguy, Cuny, Tignol and Bioulac7–Reference Menzies, Chamberlain, Laird, Thelen, Sahakian and Bullmore9], we segmented four cortical ROIs: ACC, DLPFC, OFC and the frontal pole (Fpole – anterior part of the frontal cortex − BA10, sometimes included in the OFC or DLPFC [Reference Carlen56]). As subcortical ROIs we individualised two parts of the striatum (the Caudate nucleus and Putamen) and the Thalamus. While the caudate nucleus and putamen form a single anatomo-functional unit (the striatum), they have a different location; therefore different tracts, with different shapes, connecting them to the cortex. Shape and length of a tract are important parameters in the output of quantitative tracking, thus in the comparison of tracts. Therefore, we studied these two parts of the striatum separately to obtain the most robust results.
Segmentation was performed automatically on the T1 images using FreeSurfer (https://surfer.nmr.mgh.harvard.edu/). Cortical segmentation was based on the Destrieux Atlas [Reference Destrieux, Fischl, Dale and Halgren57], subcortical segmentation on the Fischl parcellation [Reference Fischl, Salat, Busa, Albert, Dieterich and Haselgrove58]. Masks were binned at 0.5 to avoid overlap. The list of the FreeSurfer labels for each mask is reported in STable 1. The mean volume for each mask was not significantly different between groups (two-tailed t-tests, data not shown).
2.5 Probabilistic tracking
We applied the probtrackx function of FDT [Reference Behrens, Berg, Jbabdi, Rushworth and Woolrich37] with default parameters (5000 samples per voxel, path length of 2000 × 0.5 mm steps, curvature threshold of |80|° and loop-checking criteria enabled). It was run in each subject’s native space, from each seed (Caudate nucleus, Putamen and Thalamus) to each target (ACC, OFC, DLPFC and Fpole) (12 connections per hemisphere). This direction is a strictly methodological consideration and makes no assumption about the anatomical direction of the axons [Reference Jones, Knösche and Turner31, Reference Jones43]. The smallest ROI is used as the seed for maximum efficacy and robustness [Reference Behrens, Berg, Jbabdi, Rushworth and Woolrich37, Reference Bohanna, Georgiou-Karistianis and Egan47]. We only investigated ipsilateral connections, and each connection was investigated in one direction only. We checked streamlines visually for anatomical plausibility. This produced a connectivity map for each connection (i.e. 24 maps, 4 maps for each seed), in which each seed voxel had for value the number of streamlines connecting it to the target (0–5000).
2.6 Statistical analyses
2.6.1 Connectivity: spatial spread and density
Spatial spread of projections was defined as the proportion of seed voxels connected to the target: . For each of the 24 connections, we tested the difference of mean PVox between groups using permutation tests with 106 iterations and α = 5%. We corrected for multiple comparisons using a False Discovery Rate (FDR) of 10% for mathematical reasons [Reference Colquhoun59, Reference Benjamini and Hochberg60], and P-values were adjusted according to Benjamini & Hochberg [Reference Benjamini and Hochberg60–Reference Benjamini and Yekutieli62] (detailed results are also presented with FDR=5%). For OCD patients, we investigated the relationship of PVox with YBOCS, YBOCS-O, YBOCS-C, Padua3 and disease duration using Spearman’s rank correlation. Likewise, we used permutation tests and FDR correction.
Second, we investigated whether the density of connections amongst the connected voxels was modified. Indeed, if PVox is decreased but the total number of connections is unchanged, one might expect an increase in Density. Density was defined, for each connection, as the total number of streamlines from the seed to the target, divided by the number of connected seed voxels: . Statistical tests were the same as PVox.
2.6.2 Segregation of the connections
Having established the conserved topography of the connections (details in Supplementary material), we tested a difference in the segregation of cortical projections in each seedbetween OCD and controls. For each seed-voxel, we derived an index of segregation: . Higher values (max = 1) indicate high segregation, and lower values (0.25) indicate evenly distributed connections from/to the four cortical targets (Fig. 1). We tested the difference of means between groups using a permutation test and FDR correction.
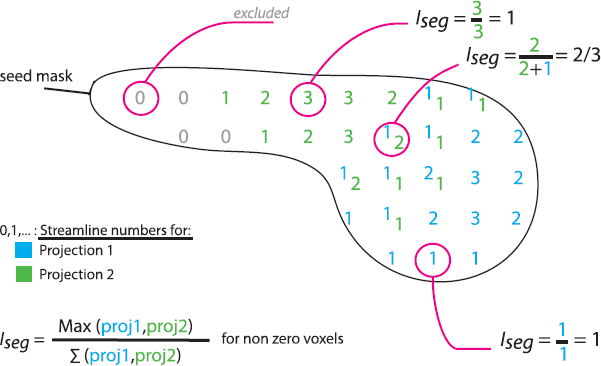
Fig. 1. Iseg, index of segregation. Schematic sagittal section of a caudate nucleus, one of the seed ROIs. Each number represents the number of streamlines made from the underlying seed voxel to region 1 (blue) or region 2 (green). Some voxels have streamlines connecting them to both region 1 and 2. Voxels with no connections (0) are excluded. Different examples of Iseg calculations are shown. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.).
All statistical analyses were performed using MATLAB (The MathWorks, Inc., MA, USA) and additional toolboxes where so indicated.
3. Results
3.1 Participants
Thirty-seven OCD patients and 37 healthy controls, matched for age and sex were included in the study (see Table 1 for demographic and clinical data). Patients had predominant checking symptoms and had a mean score of 22.49 on the YBOCS (max 40, >16 is pathological). Fifteen OCD patients had no medication, other patients received psychotropic medications which are reported, with detailed clinical scores, in STable 2.
Table 1 Demographic and clinical characteristics of the two groups.

3.2 Connectivity: spatial spread (PVox) and density
PVox for OCD patients was reduced in 12/12 connections in the left hemisphere and 7/12 in the right hemisphere compared to controls (Fig. 2, darker bars). There was a non-significant trend towards a reduction for the other connections. Detailed means, standard deviations, p-values and FDR-adjusted p-values are provided in STable 3.

Fig. 2. Connectivity changes in OCD patients. The rows correspond to each seed: Caudate nucleus, Putamen and Thalamus, left and right. An example of the segmented masks is given as row entries. The columns correspond to each target: ACC, DLPFC, OFC, and Fpole. Representative segmented masks figure as column entries. A composite of all masks is in the top left corner. Y axes represent the delta absolute value of PVox and Density for patients relative to controls. In each cell, the dark bar is the difference in PVox and the light bar is the difference in Density in patients vs. controls for that connection. Significant changes after FDR adjustment are indicated by the black triangles.
Density was reduced in OCD in 7/12 connections in the left hemisphere and 2/12 in the right hemisphere. In addition, Density was increased in OCD for the right ACC-Caudate connection compared to controls (Fig. 2, lighter bars). Detailed means, standard deviations, raw p-values and FDR-adjusted p-values are provided in STable 4.
3.3 Clinical correlations
No clinical correlation (YBOCS, YBOCS-O, YBOCS-C, Padua3 scores and duration of illness) was statistically significant after correction for multiple comparisons with any of our connectivity or segregation measures. Moreover, we identified no trend as ρ was generally small (max ρ2 =.17) and indiscriminately positive or negative.
3.4 Topography and segregation of the connections
The topography of cortical basal ganglia/thalamic connections was the same in OCD patients and controls with ACC, OFC, Fpole and DLPFC connections organised in this order (regression equations, ρ2 and P-values in Table S5).
Iseg was increased bilaterally in the putamen and thalamus in OCD compared to controls. A trend was also observed for the caudate nuclei (Table 2). This increase seemed characterised, visually, by a marked increase of subcortical voxels connected to only one cortical area (Iseg = 1, Fig. 3).
Table 2 Segregation. The segregation index Iseg ranges from 0.25 (least segregation) to 1 (most segregation, no overlap, single connection). The criterion p-value was 0.046 for an FDR of 10%.


Fig. 3. Distribution of Iseg for each seed. Values were sorted into 100 bins of width 0.01. The histogram was smoothed with a sliding boxcar, width of 0.03 X axes represent the index of segregation with higher values (max = 1) for more segregation and minimal values (min = 0.25) evenly distributed connections to the four targets. Y axes represent the number of voxels.
4. Discussion
We found altered associative and limbic cortico-basal-ganglia-thalamic anatomical connections in OCD patients compared to healthy controls: their spatial spread was reduced for 19/24 connections, so was the density of about half (9/24) of these connections. In addition, we showed that these connections were hyper-segregated in OCD.
For methodological and anatomical reasons, we did not study the connectivity of all the basal ganglia. The pallidum and the nucleus accumbens, both relevant to OCD physiopathology and part of cortico-basal ganglia circuits, were not included in the analyses. Indeed, DTI cannot access the axonal tracts connecting pallidum to striatum (they are mostly embedded in grey matter), and the pallido-thalamic connection follows a convoluted path that would not be traced accurately. Concerning the nucleus accumbens, we included it in the caudate nucleus ROI as it mostly runs underneath its ventral edge, and because there is no clear morphological criteria to isolate it from the striatum. Also, we did not perform a whole cortex analysis (motor regions included) because we wanted to base our study on strong hypotheses, and the involvement of non-prefrontal regions is inconsistent across studies and not consensual [Reference Graybiel and Rauch4–Reference Menzies, Chamberlain, Laird, Thelen, Sahakian and Bullmore9].
Concerning the tracking process, basal ganglia were identified as seeds and cortical areas as targets only for methodological reasons [Reference Behrens, Berg, Jbabdi, Rushworth and Woolrich37, Reference Bohanna, Georgiou-Karistianis and Egan47]; indeed, tractography is non-directional [Reference Jones, Knösche and Turner31, Reference Jones43]. Nonetheless, because no striato-cortical projection is known in primates, we will consider hereafter that modifications of the striato-cortical tracts reflects modifications of the cortico-striatal axonal projections.
This change of connections, demonstrated with probabilistic tractography, could provide an anatomical substrate of the dysfunctional cortico-basal-ganglia circuits in OCD. However, probabilistic tractography is an indirect estimate of axonal pathways [Reference Jones, Knösche and Turner31, Reference Behrens, Berg, Jbabdi, Rushworth and Woolrich37], based on algorithms with no anatomical priors. Thus, it could create false-positive pathways through the white matter [Reference Jones, Knösche and Turner31, Reference Dyrby, Søgaard, Parker, Alexander, Lind and Baaré35]. Here, we studied connections for which there is ample anatomical evidence [Reference Haber, Kim, Mailly and Calzavara20, Reference McFarland and Haber27] [e.g. Reference Haber, Kim, Mailly and Calzavara20, Reference McFarland and Haber27], and the pathways through the white matter were checked visually for anatomical plausibility. Moreover, the probtrackx algorithm which we used has been validated specifically for cortico-striatal and cortico-thalamic pathways by direct comparison to axonal tracing [Reference Lehman, Greenberg, McIntyre, Rasmussen and Haber63–Reference Johansen-Berg, Behrens, Sillery, Ciccarelli, Thompson and Smith66].
The streamline counts used to assess PVox and Density are also given by tracking; thereby subject to limitations. They cannot be considered as a direct estimate of the underlying number of axons [Reference Jones, Knösche and Turner31]. Indeed, the streamline count is bounded by the number of iterations of the algorithm (here 5000). Nonetheless, experimentally, the number of streamlines from the cortex has a monotonic relationship to the number of axons and neuronal bodies stained using neuroanatomical tracers [Reference Gao, Choe, Stepniewska, Li, Avison and Anderson32], or the density of axon terminals [Reference Harsan, Dávid, Reisert, Schnell, Hennig and von Elverfeldt34]. Consequently, a variation in the number of streamlines (and the indices that we derived, PVox and Density) gives a direction of the variation of the underlying number of axons, but does not quantify this difference. Thus, even if the difference cannot be precisely quantified, our results are in favour of a reduced number of axons in cortico-basal-ganglia connections in OCD.
In light of these methodological considerations, we find that our results can be trusted concerning the validity of the tracts as well as the comparison of streamline counts. Consequently, the reduction of the cortico-striatal connections in OCD demonstrated with tractography is indicative of a reduction in cortico-striatal axonal projections.
Schematically, the cortex is thought to provide voluntary control over learned behaviour stored in and implemented by the basal ganglia. Clinically, OCD patients are unable to inhibit unwanted thoughts and behaviour, even though they can identify their inappropriate character. Therefore, our results provide anatomical support for a lack of cortical voluntary control over habits stored in the basal ganglia [Reference Gillan, Papmeyer, Morein-Zamir, Sahakian, Fineberg and Robbins2, Reference Graybiel and Rauch4, Reference Schwartz5, Reference Graybiel67–Reference Gillan, Morein-Zamir, Kaser, Fineberg, Sule and Sahakian70]. One might argue that such a reduction in anatomical connections is contradictory with the increased functional connectivity reported in other studies [Reference Harrison, Pujol, Cardoner, Deus, Alonso and Lopez-Sola71, Reference Abe, Sakai, Nishida, Nakamae, Yamada and Fukui72]; however, this increased functional connectivity could be a homeostatic attempt to counterbalance the lack of connections.
Among the connectivity parameters, the one that changed for the most connections was the spatial spread (PVox). The difference between the two measurements might be explained by the wider variance of Density, but could also suggest that the spatial organisation of connections is closer to the core of the pathophysiology.
Indeed, in OCD patients we also found an alteration in the organisation of the cortico-basal-ganglia anatomical connections in the form of a hyper-segregation of the cortical projection fields.
Our segregation index, Iseg, uses the connectivity profiles of voxels in each seed (the relative strengths of each connection for that voxel). Such connectivity profiles have been used in other studies to make connectivity-based parcellations of the striatum and cortex [Reference Graybiel67, Reference Bogacz and Larsen69, Reference Gillan, Morein-Zamir, Kaser, Fineberg, Sule and Sahakian70]. These parcellations are in agreement with both axonal tracing in non-human primates, and functional data in humans. Thus, connectivity profiles appear to contain anatomically and functionally relevant information. With Iseg, we used the same connectivity profiles but instead focused on the information about overlap and segregation which they contain.
Partial overlap of neighbouring cortico-basal-ganglia-thalamic loops is a crucial component of their anatomical and functional organisation [Reference Haber, Kim, Mailly and Calzavara20, Reference Haynes and Haber22, Reference McFarland and Haber27]. It allows the convergence and integration of different types of information, which in turn allows a fine-tuning of behavioural output [Reference Frank68, Reference Bogacz and Larsen69]. According to models of behavioural selection, each specific combination of cortical inputs − representing an environmental state – elicits activity in a specific striatal cell assembly [Reference Carrillo-Reid, Tecuapetla, Tapia, Hernandez-Cruz, Galarraga and Drucker-Colin73], and thus a specific behaviour [Reference Frank68, Reference Bogacz and Larsen69].
Here, we demonstrate an increased segregation of the associative and limbic prefrontal inputs to the striatum in OCD. It follows that their functional integration would also be disturbed in OCD. Because this integration is used to control reinforcement learning [Reference Graybiel67, Reference Costa74] and behavioural selection in the basal ganglia [Reference Bogacz and Larsen69], its disturbance could explain why OCD patients select inappropriate programs for the circumstances. This may be reflected in patients’ inability to use new conditions to update behaviour [Reference Gillan, Morein-Zamir, Urcelay, Sule, Voon and Apergis-Schoute1, Reference Figee, Vink, de Geus, Vulink, Veltman and Westenberg11], leading to the repetition of compulsions and cognitive rigidity [Reference Bradbury, Cassin and Rector3]. Thus, hyper-segregation provides working hypotheses to be tested in future functional and behavioural studies.
Reduced connectivity and hyper-segregation were also found in prefrontal-thalamic connections. As tractography is non-directionnal, this can reflect modifications in cortico-thalamic and/or thalamo-cortical axons. The involvement of the thalamus and its cortical connections in OCD has been described in functional studies [Reference Menzies, Chamberlain, Laird, Thelen, Sahakian and Bullmore9]. One might consider a dysregulation of the balance between the cortico-basal ganglia (supporting implicit/automatic function) and the cortico-thalamic circuit (processing explicit information) in OCD [Reference Graybiel and Rauch4]. One might also consider that the hypersegregation is ‘simply’ carried-on from the cortical-basal ganglia projection throughout the loop. More studies are needed to understand the implication of these two loops in the expression of OCD symptoms.
It would also be of interest to identify the respective role of anatomical alterations in the associative and the limbic loops, in OCD. However, for methodological reasons, we cannot compare the modification of anatomical connectivity between two loops. Indeed, the magnitude of the difference in PVox or Density cannot be compared between different anatomical connections because streamline counts are influenced by the shape and length of the tract (e.g. one cannot conclude that PVox is more reduced for the left caudate-ACC connection than for the left thalamus-OFC connection) [Reference Jones, Knösche and Turner31, Reference Behrens, Woolrich, Jenkinson, Johansen-Berg, Nunes and Clare36].
While we showed a modification of connections in cortico-basal-ganglia-thalamic loops in OCD, we found no correlation between our connectivity measures and the clinical characteristics of the patients. This could be due partially to the non-linear relationship of streamlines and axon counts [Reference Gao, Choe, Stepniewska, Li, Avison and Anderson32], but also to the homogeneity of the population in terms of severity. Our OCD group was also homogenous concerning clinical subtype. As neurophysiological differences have been shown between OCD subtypes [Reference Mataix-Cols, Wooderson, Lawrence, Brammer, Speckens and Phillips75], the inclusion was limited to patients with predominantly checking symptoms. This limits the application of our results to other clinical subtypes; however, checking is one of the most common symptoms in OCD, and dysfunctions of the associative and limbic part of cortico-basal-ganglia circuits (in fMRI) are shared by all the major subtypes [Reference Mataix-Cols, Wooderson, Lawrence, Brammer, Speckens and Phillips75].
In conclusion, we used probabilistic tractography to demonstrate that the limbic and associative prefrontal-striatal and prefrontal-thalamic loops are anatomically altered in OCD. More specifically, we found a reduction in the spatial extent and density of connections, and a hyper-segregation of the cortical connections with the basal ganglia in OCD. We hypothesise that these quantitative and qualitative differences in anatomical connections might lead to a reduced and ineffective regulation by associative and limbic prefrontal areas of habit learning and expression by the basal ganglia in patients. Clinically, cognitive and behavioural therapy might act through a reorganisation of these connections, by driving plasticity during the reprogramming of pathological habits for appropriate ones [Reference Schwartz5].
Financial support
This work was funded by a Partenariat Institution Citoyen Recherche et Innovation (PICRI) from the Région Île de France, and a grant from the Agence Nationale de la Recherche (ANR): ANR-06-NEURO-006-01 BG EMO/PATH 2006-2010. It also benefited from the French Program ‘Investissements d’Avenir’ ANR-10-IAIHU-06.
WIH received a doctoral grant from the French Ministry for Research administered by the Université Paris Descartes.
Conflict of interest
None.
Acknowledgments
We wish to thank Dr Mike Sharman and Dr Arnaud Messé for their technical advice, Dr Bruno Falissard for his opinion on the statistical analyses and Dr Karim N'Diaye for his helpful comments on the manuscript.
Appendix A Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.eurpsy.2018.01.005.








Comments
No Comments have been published for this article.