Over the past three decades, there has been much effort towards identifying neurobiological abnormalities in schizophrenia. Part of the appeal of neuroimaging is that it bridges the gap between psychiatry and the rest of medicine, and offers the potential for applying objective biological markers (biomarkers), rather than clinical history alone, to the diagnosis and clinical management of the illness. Nevertheless, despite three decades of research, neuroimaging has been limited in the extent to which it has improved our understanding of the pathophysiology of schizophrenia and is yet to have a major impact clinically.
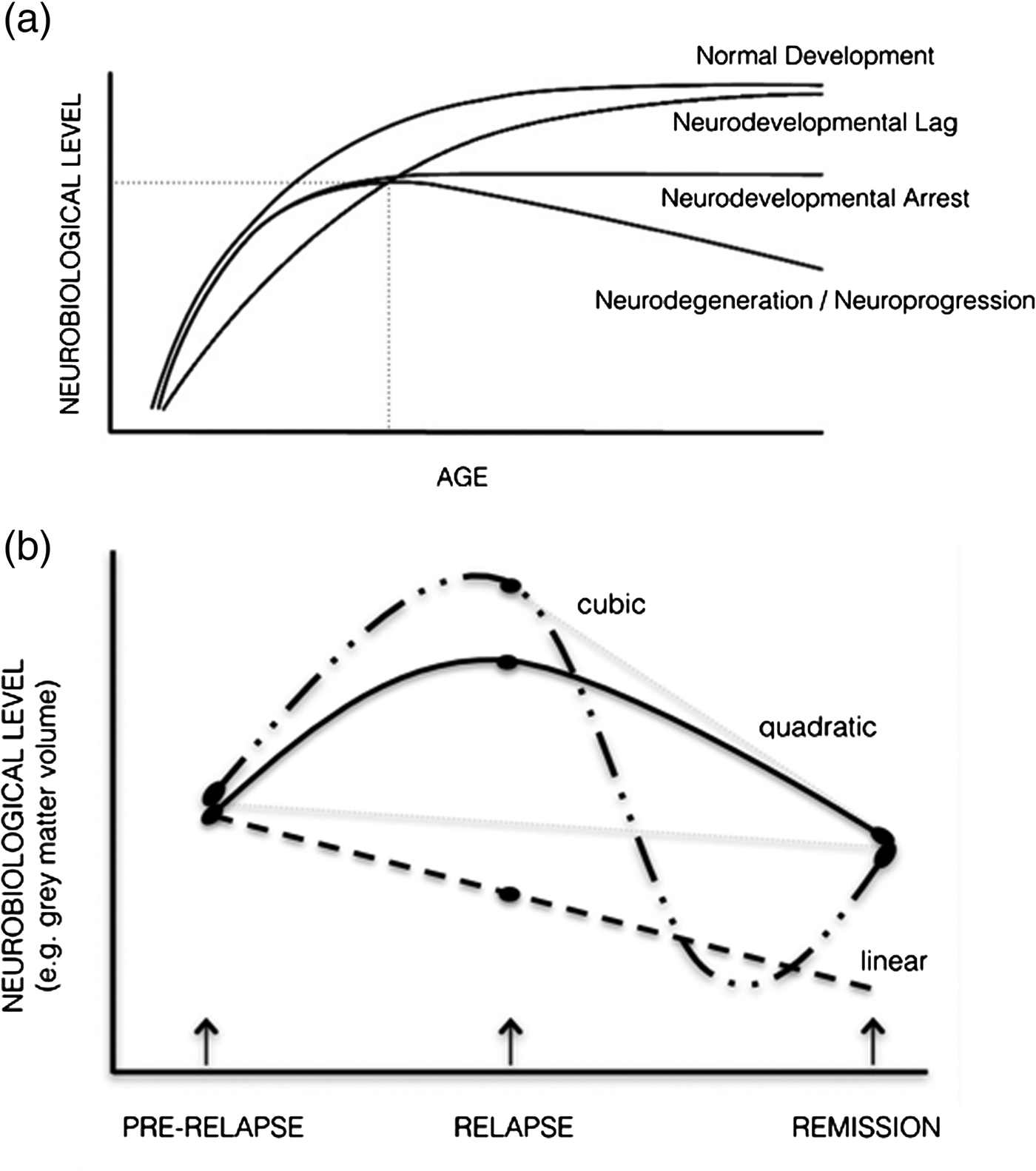
The large bulk of neuroimaging studies in schizophrenia and psychosis have involved cross-sectional comparisons between patients at various illness stages and healthy controls at a single point in time. Such studies have typically assessed regional grey matter or whole brain volume, although more recent studies have examined functional (i.e., functional magnetic resonance imaging (fMRI)) and neurochemical alterations including in N-acetyl-aspartate (NAA) (Kraguljac et al. Reference Kraguljac, Reid, White, Jones, Den Hollander, Lowman and Lahti2012), glutamate (GLU) and dopamine (Urban & Abi-Dargham, Reference Urban and Abi-Dargham2010). Although abnormalities in these systems have been identified, the use of single assessments provides a simple snapshot in time and ignores the importance of the trajectory of change (Pantelis et al. Reference Pantelis, Yucel, Bora, Fornito, Testa, Brewer, Velakoulis and Wood2009). The importance of assessing the course of change is illustrated in Fig. 1a, in three hypothetical neurobiological (e.g., cognitive, imaging) outcomes (figure modified from Testa & Pantelis, Reference Testa, Pantelis, Wood, Allen and Pantelis2009). For instance, patients and controls may not differ on a cognitive or neuroimaging outcome at a particular time point, despite each having a distinct developmental course (e.g., lag, arrest, degeneration). Furthermore, the timing of the imaging scan may influence the degree of difference in a neuroimaging outcome between patients and controls. Although individuals may follow the same ‘course’, they could have very different outcomes if they were all assessed at various points along their trajectory. We suggest that the analysis of brain trajectories may be more informative in delineating different populations of individuals with schizophrenia or other psychoses and may inform our understanding of these illnesses, and the effect of treatment. Specifically, we propose that longitudinal designs in relation to changes in clinical status and treatment be employed to investigate neuroimaging markers of the course of psychosis particularly in the context of relapse and remission.

Fig. 1. (a) Schematic depicting possible neurodevelopmental trajectories relevant to neurobiological indices during maturation. At the point of intersection of dotted lines, individuals with very different developmental trajectories have the same degree of impairment (figure modifed from Testa & Pantelis, Reference Testa, Pantelis, Wood, Allen and Pantelis2009). (b) Schematic representing the types of models that may best characterise a neurobiological marker associated with change in clinical state (relapse and remission from the acute episode). Note that if only two time points were assessed, the neurobiological change from remission of the acute episode (depicted by the dashed grey lines) would be different depending if the scan was initially assessed at its peak level (resulting in a steep decline) or before its peak (resulting in no change).
Trajectories of change can inform neurodevelopment and neuroprogression of the illness
The approach of performing longitudinal imaging to assess brain trajectories is exemplified in the developmental studies of childhood-onset schizophrenia (COS) and in mapping normal trajectories of brain development (Gogtay, Reference Gogtay2008). Longitudinal imaging studies assessing the normal trajectory of brain changes occurring in different brain regions have demonstrated a parietal–frontal progression of grey matter loss and regionally circumscribed non-linear trajectories during normal development (Thompson et al. Reference Thompson, Vidal, Giedd, Gochman, Blumenthal, Nicolson, Toga and Rapoport2001; Gogtay et al. Reference Gogtay, Giedd, Lusk, Hayashi, Greenstein, Vaituzis, Nugent III, Herman, Clasen, Toga, Rapoport and Thompson2004; Lenroot & Giedd, Reference Lenroot and Giedd2006; Shaw et al. Reference Shaw, Kabani, Lerch, Eckstrand, Lenroot, Gogtay, Greenstein, Clasen, Evans, Rapoport, Giedd and Wise2008). Furthermore, longitudinal studies of cortical grey matter in COS patients and their siblings have provided important insights into the effect of early illness onset on specific developmental windows. In COS probands, during the adolescent period the normal parietofrontal pattern of progressive grey matter loss is more severe, but post-adolescence the grey matter loss slows down and becomes localised to prefrontal and temporal cortices (Rapoport et al. Reference Rapoport, Giedd, Blumenthal, Hamburger, Jeffries, Fernandez, Nicolson, Bedwell, Lenane, Zijdenbos, Paus and Evans1999; Thompson et al. Reference Thompson, Vidal, Giedd, Gochman, Blumenthal, Nicolson, Toga and Rapoport2001; Greenstein et al. Reference Greenstein, Lerch, Shaw, Clasen, Giedd, Gochman, Rapoport and Gogtay2006; Gogtay et al. Reference Gogtay, Lu, Leow, Klunder, Lee, Chavez, Greenstein, Giedd, Toga, Rapoport and Thompson2008). Healthy siblings of COS patients also show grey matter deficits in early ages but these appear to normalise by late adolescence. These findings suggest that there is a ‘developmental lag’ (see Fig. 1a) of cortical grey matter volumes in a genetic at-risk group for psychosis that is close to the developmental roots of psychosis. There are several possible explanations for these patterns of grey matter deficits in COS and their siblings, including notions related to ‘age-specific’ endophenotypes and protective (resilience) factors (see Pantelis et al. Reference Pantelis, Yucel, Bora, Fornito, Testa, Brewer, Velakoulis and Wood2009; Gogtay et al. Reference Gogtay, Vyas, Testa, Wood and Pantelis2011; Pantelis & Bartholomeusz, in press). Importantly, however, the above longitudinal studies provide opportunities to examine trajectories of brain changes in relation to an early onset of schizophrenia and early brain maturational stage.
The effect of psychosis onset and early illness on brain structural changes has also been documented by the longitudinal studies in clinical and genetic ‘at-risk’ samples and over the first years of illness. In contrast to the pre-pubertal onset of psychosis of COS individuals, these samples typically present during adolescence, which affords the opportunity to examine the effect of illness onset during a later brain maturational window. These studies have revealed progressive grey matter reductions in select frontal and temporal regions at psychosis onset and over the initial stages of illness (Pantelis & Wood, Reference Pantelis and Wood2009; Wood et al. Reference Wood, Pantelis, Yung, Velakoulis and Mcgorry2009). Regional grey matter reductions have been reported to occur at double the rate seen in normal adolescence (Sun et al. Reference Sun, Phillips, Velakoulis, Yung, Mcgorry, Wood, Van Erp, Thompson, Toga, Cannon and Pantelis2009a, Reference Sun, Stuart, Jenkinson, Wood, Mcgorry, Velakoulis, Van Erp, Thompson, Toga, Smith, Cannon and Pantelisb) and at a faster rate at the earliest rather than later stages of illness (reviewed in Wood et al. Reference Wood, Pantelis, Yung, Velakoulis and Mcgorry2009). The faster rate of progressive change is evidenced in the two summary figures demonstrating more severe grey matter loss in the superior temporal gyrus and insula cortices during transition to psychosis for the first time and over the initial 2–4 years of illness (Fig. 2a, b).

Fig. 2. (a) Longitudinal MRI volume changes in left PT across the stages of psychosis (similar changes in other regions of superior temporal gyrus; left > right). MRI, magnetic resonance imaging; PT, planum temporale; %/year, mean annualised change per year; T1, time 1 MRI scan; T2, time 2 MRI scan; UHR-NP, ultra high risk non-psychotic; UHR-P, ultra high risk psychotic; FEP, first-episode psychosis; Sz, schizophrenia (Takahashi et al. Reference Takahashi, Wood, Soulsby, Kawasaki, Mcgorry, Suzuki, Velakoulis and Pantelis2009a, Reference Takahashi, Wood, Yung, Soulsby, Mcgorry, Suzuki, Kawasaki, Phillips, Velakoulis and Pantelise, Reference Takahashi, Wood, Kawasaki, Suzuki, Velakoulis and Pantelis2010b, Reference Takahashi, Wood, Yung, Walterfang, Phillips, Soulsby, Kawasaki, Mcgorry, Suzuki, Velakoulis and Pantelisc). (b) Longitudinal MRI volume changes in left anterior insula across the stages of psychosis (similar changes in other insula regions). MRI, magnetic resonance imaging; %/year, mean annualised change per year; T1, time 1 MRI scan; T2, time 2 MRI scan; UHR-NP, ultra high risk non-psychotic; UHR-P, ultra high risk psychotic; FEP, first-episode psychosis; Sz, schizophrenia (Takahashi et al. Reference Takahashi, Wood, Soulsby, Mcgorry, Tanino, Suzuki, Velakoulis and Pantelis2009b, Reference Takahashi, Wood, Soulsby, Tanino, Wong, Mcgorry, Suzuki, Velakoulis and Pantelisc, Reference Takahashi, Wood, Yung, Phillips, Soulsby, Mcgorry, Tanino, Zhou, Suzuki, Velakoulis and Pantelisd).
The above findings raise several considerations. First, they illustrate the importance of placing the above brain changes in the context of the normal neurodevelopmental trajectory. Second, they suggest that the brain changes in schizophrenia psychoses may be occurring in a non-linear fashion, characterised by active changes at the onset and earliest stages of the illness. Third, as the above longitudinal imaging studies were limited to only two time points, the nature of the brain changes occurring with illness onset remains unclear, including whether such changes are inherently progressive. A re-conceptualisation of neuroimaging designs is needed to assess these potential non-linear patterns of brain changes that may arise throughout the illness, particularly in the context of a changeable clinical picture.
Linking longitudinal imaging with changes in acute clinical status
Although mapping brain trajectories within a development context is important, the above studies remain largely removed from the clinical manifestations of the illness. Thus, a few studies have specifically linked neuroimaging abnormalities with changes in clinical status or outcome. Although several structural and functional markers have been identified as promising predictors of later transition to psychosis (see meta-analysis by Smieskova et al. Reference Smieskova, Fusar-Poli, Allen, Bendfeldt, Stieglitz, Drewe, Radue, Mcguire, Riecher-Rossler and Borgwardt2010), there are a few neuroimaging correlates of other clinical changes, particularly outside of the prodromal period. Of such studies, most involve a single neuroimaging assessment with clinical follow-up rather than combined clinical/imaging longitudinal approaches. For instance, Bodnar et al. (Reference Bodnar, Achim, Malla, Joober, Benoit and Lepage2012) identified differential neural activation between individuals with first-episode schizophrenia who later achieved clinical remission compared to those who did not. Although influential in its paradigm to link neuroimaging with clinical status, change in functional activity over time was not examined. Thus, it is unknown whether neural activity changes as a function of remission status. Some longitudinal structural MRI studies have reported associations between volume loss and certain features of clinical course. Volumetric reduction in whole brain, whole grey matter and certain regions including the putamen, prefrontal and superior temporal cortex have shown associations with greater clinical symptom severity (based on total, positive and negative scores) (Mathalon et al. Reference Mathalon, Sullivan, Lim and Pfefferbaum2001; Takahashi et al. Reference Takahashi, Suzuki, Zhou, Tanino, Nakamura, Kawasaki, Seto and Kurachi2010a), a differential pattern of clinical improvement related to different symptom dimensions (Gur et al. Reference Gur, Cowell, Turetsky, Gallacher, Cannon, Bilker and Gur1998), and associations with ‘poor-outcome’ patients based on the dichotomy of longitudinal clinical parameters into good and poor-outcome subgroups (Mitelman et al. Reference Mitelman, Canfield, Chu, Brickman, Shihabuddin, Hazlett and Buchsbaum2009). However, such findings are not typically reproducible and many have been investigated on a post hoc basis. The long follow-up periods (1–5 years) typically employed in previous studies also precludes the characterisation of acute clinical changes that may be important in mediating specific brain/clinical relationships. Therefore, imaging paradigms that specifically link neuroimaging changes with changes in clinical status over a short period may offer valuable insight into the neurobiology underlying specific dimensions of the illness.
Interrogating active phases of psychotic illness
There are important parallels between the brain structural and clinical trajectories of schizophrenia. The progressive, yet non-linear, brain changes observed at the earliest stages of psychotic illness suggest that the onset and early stage of schizophrenia psychoses is a critical time in the illness, whereby active brain changes are taking place. Similar to these progressive brain changes, the prodrome and early stage of illness is often characterised by a deterioration in symptoms and functioning that tends to plateau at later stages (Lieberman et al. Reference Lieberman, Perkins, Belger, Chakos, Jarskog, Boteva and Gilmore2001). Clinically, a proportion of individuals with schizophrenia who have a relapse of their illness fail to return to their premorbid level of functioning (Lieberman et al. Reference Lieberman, Perkins, Belger, Chakos, Jarskog, Boteva and Gilmore2001), potentially indicating ongoing progressive brain changes associated with each repeated relapse of illness. Consequently, the identification of neurobiological processes or ‘biomarkers’ associated with continuing illness and particularly active periods of illness (the prodromal phase, initial episode and subsequent relapses) is important to characterise. As a psychotic episode will typically resolve over several weeks to months, neurobiological changes can be tracked over a relatively short period of time.
We recently reviewed the literature investigating neurobiological markers of active illness and specifically illness relapse (Cropley et al. Reference Cropley, Wood and Pantelis2013). Candidate markers of active psychotic state include striatal hyperdopaminergia in the associative striatum (measured with positron emission tomography (PET) and single-photon emission computed tomography (SPECT) imaging), GLU abnormalities indexing excitotoxicity, whole brain or frontotemporal brain structural changes and neuroimaging indices of an active immune or pathophysiological process. Most of these markers have been inferred from cross-sectional and correlational approaches, and require further validation longitudinally. Some markers, such as striatal hyperdopaminergia, may show both trait and state features (reviewed in Cropley et al. Reference Cropley, Wood and Pantelis2013), whereas others such as GLU abnormalities may vary with symptom severity rather than positive psychotic symptomatology per se (Egerton et al. Reference Egerton, Brugger, Raffin, Barker, Lythgoe, Mcguire and Stone2012). In structural brain morphology, progressive changes have been noted to occur with active psychotic illness. Cahn et al. (Reference Cahn, Rais, Stigter, Van Haren, Caspers, Hulshoff Pol, Xu, Schnack and Kahn2009) reported greater global grey matter loss with longer duration of active psychosis, although with this design it is unclear whether the brain changes are related to continuous psychotic symptoms or an episodic course. Garver et al. (Reference Garver, Nair, Christensen, Holcomb and Kingsbury2000) have systematically assessed longitudinal brain structural changes associated with illness exacerbation and recovery. They reported that global brain volume increases and ventricular volume reductions were associated with an exacerbation of psychosis and the reverse relationship was seen with remission. These findings suggest that volumetric increases (postulated to reflect brain swelling) may accompany acute psychosis, which reverse with illness remission. Such a relationship associated with illness acuity is informative, as it suggests a fluctuating course of brain changes with changes in clinical state. Therefore, longitudinal designs that best characterise more complex trajectories would be useful to employ.
Frequent serial neuroimaging across discrete clinical stages is needed
The Garver et al. (Reference Garver, Nair, Christensen, Holcomb and Kingsbury2000) finding demonstrating brain expansion and reduction with psychosis exacerbation and remission is important as it demonstrates that the nature of the neurobiological change may relate to the timing of the imaging scans relative to the state of illness assessed. The above structural findings also challenge the interpretation of previous longitudinal brain volume studies and the usefulness of current longitudinal approaches. Controversially, the state-related volumetric changes assessed near the peak of brain expansion may also account for the faster rate of brain volumetric reductions that have been previously identified in schizophrenia. Therefore, even longitudinal designs using two time points are insufficient for detecting possible non-linear brain changes, and how these relate to psychotic symptom changes. Frequent imaging assessments are needed to model more complex trajectories (e.g., quadratic, cubic and quartic) occurring with acute symptom changes. In Fig. 1b, various alternative possible trajectories associated with state changes are depicted, indicating that two time points assessing neurobiological indices are not adequate to determine the nature of the trajectories involved.
Importantly, these more complex trajectories should be delineated in relation to well-characterised symptom profiles. Two recent studies illustrate the need to coordinate the neuroimaging assessment with clinical status. Bodnar et al. (Reference Bodnar, Achim, Malla, Joober, Benoit and Lepage2012) identified positive neural activation during memory encoding in individuals with first-episode schizophrenia who did not achieve remission at 1 year while those who did achieve remission showed negative activation. However, in this study the imaging scan was not assessed when remission status was determined. Nearly 40% of individuals in the non-remitted group were in a remitted state at the time of their fMRI scan, suggesting that there was considerable variability in clinical status over time. Given potential state-related brain changes, it is thus possible that activation patterns in some individuals who later achieved remission status were different at the time of the scan. This could have reduced the sensitivity for detecting further activation differences related to specific clinical features (e.g., episodic v. continuous course). In contrast, in the magnetic resonance spectroscopy (MRS) study by Egerton et al. (Reference Egerton, Brugger, Raffin, Barker, Lythgoe, Mcguire and Stone2012), the symptom assessment used to allocate patients to clinical status group (symptomatic exacerbation v. symptomatic remission) was taken at the time of imaging. This study found increased Glu/creatine levels in the anterior cingulate in individuals who were still symptomatic compared to those in remission. This paradigm is useful as it controlled for differences in symptom profile between individuals, allowing a more precise investigation of brain function in relation to clinical status. Subsequent paradigms that include a serial, longitudinal approach will be able to elucidate any short-term changes associated with symptomatic remission (or no remission) in the same subjects.
The effect of stress, drugs and inflammation
Although brain changes across the stages of schizophrenia are well documented, an inherent limitation of neuroimaging is that it cannot elucidate the mechanisms that may underlie such changes. A number of factors may affect brain trajectories seen in acute schizophrenia, including medication, substance use, lifestyle, genetics, stress and inflammatory processes. The effect of these factors, particularly antipsychotic medication, is controversial. Other factors may only contribute to brain changes at specific stages of the illness or at specific developmental windows. For instance, the increases in brain volume with illness exacerbation (see above), interpreted to reflect brain ‘swelling’, may point to active primary disease processes. An intriguing hypothesis is that such swelling may be related to neuroinflammatory processes during active psychosis or relapse (see Cropley et al. Reference Cropley, Wood and Pantelis2013). Similarly, stress and hypothalamic–pituitary–adrenal–axis function may contribute to such changes, as supported by enlarged pituitary volume prior to transition to psychosis (Garner et al. Reference Garner, Pariante, Wood, Velakoulis, Phillips, Soulsby, Brewer, Smith, Dazzan, Berger, Yung, Van Den Buuse, Murray, Mcgorry and Pantelis2005). While a detailed assessment of such factors is beyond the scope of this paper, primary and secondary illness factors should be considered in the interpretation of brain changes over the course of the illness.
A guide for future imaging work
To date, a few brain markers have been adequately assessed over time and in cohorts associated with discrete clinical phases, e.g., prospectively assessed for illness relapse. Although some markers (e.g., NAA) (Bustillo et al. Reference Bustillo, Rowland, Jung, Brooks, Qualls, Hammond, Hart and Lauriello2008) have been measured repeatedly, to date no studies have assessed the trajectory of neurobiological markers specifically associated with a psychotic episode and subsequent remission. The challenge for future research is to characterise the nature of brain trajectories (e.g., linear v. non-linear; acute v. gradual) occurring across the stages and phases of schizophrenia psychoses as well as in the context of neurodevelopment. Ultimately, it is hoped that with this approach it will be possible to identify discrete neuroimaging markers sensitive to developmental and clinical status. Such paradigms may identify markers associated with persistent or acute symptomatology, markers that do not vary over time, or markers that do change irrespective of clinical status. Other markers may not be closely related to clinical status in terms of symptoms, but represent markers of aberrant brain maturational trajectories which may have relevance to illness features that affect overall functional outcome, such as social cognition, neurocognitive function and emotional reactivity. With respect to psychosis relapse, we propose that with serial longitudinal imaging coupled with well-characterised clinical samples of illness relapse and recovery, the neurobiological trajectory indicative of a ‘relapse signature’ for psychosis could be identified.
Financial Support
This research was supported by grants from the National Health and Medical Research Council of Australia (Program Grant, ID: 566529; Project Grant, ID: 1065742) and a NARSAD Distinguished Investigator Award (Brain & Behavior Research Foundation, US) to CP. VC was supported by a NHMRC Training Fellowship (ID: 628880). CP was supported by a NHMRC Senior Principal Research Fellowship (ID: 628386).
Conflict of Interest
None.