Introduction
Mortality risks associated with physical illnesses, especially cardiometabolic health, in patients with schizophrenia are attracting increased attention (Mitchell et al., Reference Mitchell, Vancampfort, Sweers, van Winkel, Yu and De Hert2013; Carney et al., Reference Carney, Cotter, Bradshaw, Firth and Yung2016). By contrast, the periodontal health of patients with schizophrenia has remained overlooked (Arnaiz et al., Reference Arnaiz, Zumárraga, Díez-Altuna, Uriarte, Moro and Pérez-Ansorena2011; Wey et al., Reference Wey, Loh, Doss, Abu Bakar and Kisely2016). Compared with the general population, patients with schizophrenia have a high incidence of periodontal disease (Kenkre and Spadigam, Reference Kenkre and Spadigam2000; Ramon et al., Reference Ramon, Grinshpoon, Zusman and Weizman2003; Hu et al., Reference Hu, Chou, Wen, Hsieh, Tsai, Yang, Yang and Lin2016). Although periodontal disease is not an acutely life-threatening disease, an increasing body of evidence supports its association with cardiovascular disease, stroke, metabolic syndrome, pulmonary disease and adverse pregnancy outcomes (Pihlstrom et al., Reference Pihlstrom, Michalowicz and Johnson2005; Cullinan and Seymour, Reference Cullinan and Seymour2013).
Periodontal disease – including gingivitis and periodontitis – is inflammation of the periodontium due to a persistent bacterial infection that leads to the breakdown of connective tissue and bone – a major cause of tooth loss in adults (Pihlstrom et al., Reference Pihlstrom, Michalowicz and Johnson2005; Thomson et al., Reference Thomson, Sheiham and Spencer2012; Ji et al., Reference Ji, Choi and Choi2015). In the general population, recognised risk factors for periodontal disease include aged, male sex, race, unhealthy lifestyle (poor oral hygiene, smoking and alcohol use), poor nutrition (inadequate dietary consumption of calcium and vitamin D), systemic diseases (obesity, metabolic syndrome, osteoporosis, diabetes mellitus and HIV/AIDS), psychosocial stress, genetic factors and medications (Albandar, Reference Albandar2002; Pihlstrom et al., Reference Pihlstrom, Michalowicz and Johnson2005; Thomson et al., Reference Thomson, Sheiham and Spencer2012; Genco and Borgnakke, Reference Genco and Borgnakke2013). Antipsychotics and other medications affect salivary secretion often causing hyposalivation or hypersalivation. Salivary secretion dysfunction aggravates periodontal disease (Sekine et al., Reference Sekine, Rikihisa, Ogata, Echizen and Arakawa1999; Hashimoto et al., Reference Hashimoto, Uno, Miwa, Kurihara, Tanifuji and Tensho2012; Eltas et al., Reference Eltas, Kartalcı, Eltas, Dündar and Uslu2013). Only a few studies have assessed the link between antipsychotics and periodontal disease in patients with schizophrenia to date (Gopalakrishnapillai et al., Reference Gopalakrishnapillai, Iyer and Kalantharakath2012; Eltas et al., Reference Eltas, Kartalcı, Eltas, Dündar and Uslu2013). Therefore, the current study investigated this link.
Most research on periodontal disease and schizophrenia has been restricted to cross-sectional designs and small-to-medium samples; they have also focused on chronic schizophrenia (Arnaiz et al., Reference Arnaiz, Zumárraga, Díez-Altuna, Uriarte, Moro and Pérez-Ansorena2011; Gurbuz et al., Reference Gurbuz, Alatas, Kurt, Dogan and Issever2011; Teng et al., Reference Teng, Su, Chang and Lai2011; Gopalakrishnapillai et al., Reference Gopalakrishnapillai, Iyer and Kalantharakath2012; Eltas et al., Reference Eltas, Kartalcı, Eltas, Dündar and Uslu2013; Shetty and Bose, Reference Shetty and Bose2014; Nayak et al., Reference Nayak, Singh and Kota2016; Wey et al., Reference Wey, Loh, Doss, Abu Bakar and Kisely2016). The literature cannot fully explain the progress of development of periodontal disease in individuals with schizophrenia, especially the early stages of development. Given the lack of extensive literature, we identified a population-based cohort of patients newly diagnosed with schizophrenia from the Taiwan National Health Insurance Research Database (NHIRD), determined how many developed periodontal diseases within 1 year of their diagnosis, and identified risk factors associated with periodontal disease.
Methods
Study source and participants
Detailed descriptions of the Taiwan NHIRD sample and study procedures have previously been published (Hu et al., Reference Hu, Chou, Wen, Hsieh, Tsai, Yang, Yang and Lin2016; Lin et al., Reference Lin, Tsai, Wu, Chang, Wen, Liu and Lung2018). In summary, we employed the 1995–2010 NHIRD data, a subset composed of 1 million randomly sampled beneficiaries drawn in 2000. The Internal Review Board approved the study and informed consent was waived because we used de-identified medical information from the NHIRD.
We performed a cohort study of patients who were newly diagnosed with schizophrenia between 1 January 2000 and 31 December 2009. The patients were diagnosed based on the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) code 295 by at least two psychiatrists and such patients had been treated with antipsychotics for more than 3 months. The index date was defined as the date of the schizophrenia diagnosis. Exclusion criteria were as follows: HIV/AIDS, diabetes mellitus, chronic pulmonary disease, osteoporosis and alcoholism before the index date (because of the potential confounding factors for periodontal disease) (Albandar, Reference Albandar2002; Pihlstrom et al., Reference Pihlstrom, Michalowicz and Johnson2005; Hu et al., Reference Hu, Chou, Wen, Hsieh, Tsai, Yang, Yang and Lin2016). Additionally, a 2-year history of periodontal disease before the index date was recorded.
Incidence of treated periodontal disease after their index date
Claim records of periodontal disease of the participants comprised both the periodontal disease-related diagnoses (ICD-9-CM codes 523.0–523.5, 523.8 and 523.9) and anatomical therapeutic chemical codes diagnosed by dentists (91001C, 91003C, 91004C, 91006C–91008C, 91009B, 91010B, 91011C–91013C, 91104C, 92033C, P4001C and P4002C). The incidences of periodontal disease in participants diagnosed with and treated for periodontal disease within 1 year after their index date were recorded. We followed up all participants for 1 year until a diagnosis of periodontal disease, end of follow-up in medical records, death or the end of 2010.
Exposure to antipsychotics and other medications
Appendix Table A1 presents antipsychotics and other medications in this study. We acquired these medication data from prescription files and estimated the pharmacotherapy duration based on the dosing regimen of each participant and the number of units dispensed. All medications were classified into four categories: first-generation antipsychotics (FGAs), second-generation antipsychotics (SGAs), anticholinergics and antihypertensives. Exposure to antipsychotics and other medications was recorded if a participant was prescribed the same medication for at least 4 weeks during the 1-year follow-up. Co-medications were considered as concomitant drugs that were simultaneously prescribed with antipsychotics and other medications.
The risk of periodontal disease for each antipsychotic and other medication was evaluated to identify adverse effects (hyposalivation and hypersalivation) following usage. The adverse effects of continuous medication use on periodontal disease were assessed. The potential adverse effects of antipsychotics and other medications are listed in Appendix Table A2 (Friedlander and Marder, Reference Friedlander and Marder2002; Scully and Bagan, Reference Scully and Bagan2004; Muench and Hamer, Reference Muench and Hamer2012; Vinayak et al., Reference Vinayak, Annigeri, Patel and Mittal2013).
Statistical analysis
All statistical analyses were performed using SAS statistical software (v 9.1, SAS Institute, Cary, NC, USA). Patients with schizophrenia who did and did not develop periodontal disease were analysed using the Pearson's χ 2 test after stratification by sex, age, geographical region, income level, medical prescriptions and a 2-year history of periodontal disease. To analyse the independent effect of schizophrenia on the risk of developing periodontal disease, we used logistic regression after adjustment for sex, age, geographical region, income level, concomitant medical prescriptions, 2-year history of periodontal disease, potentially associated risk factors and index date. Moreover, we performed hierarchical logistic modelling using SAS GLMMIX to mitigate potential collinearity among sexes, geographical region and income level (Dai et al., Reference Dai, Li and Rocke2006). We used logistic regressions for evaluating the individual odds ratios (ORs) of periodontal disease related to the adverse effects of antipsychotics and other medications and potentially associated risk factors. A two-sided statistical significance level of p < 0.05 was used in all analyses.
Results
During the study period, 3610 patients were newly diagnosed with schizophrenia. The mean age at presentation was 34.7 years (standard deviation [s.d.] 14.3). Overall, 3295 (91.3%) patients belonged to a low income level class, and 2058 (57.0%) were prescribed multiple antipsychotics. Of all the enrolled patients, 367 (10.2%) had a 2-year history of periodontal disease before their respective index date (Table 1). During the 1-year follow-up period, 2373 (65.7%) patients received periodontal disease treatment: 300 had a 2-year history of periodontal disease and 2073 exhibited no such history (Table 2).
Table 1. Baseline characteristics of patients newly diagnosed with schizophrenia between 2000 and 2009 (n = 3610)

s.d., standard deviation; NT$, New Taiwan dollar.
a Including antipsychotic polypharmacy.
Table 2. Occurrence of treated periodontal disease among patients with newly diagnosed schizophrenia during 1-year follow-up
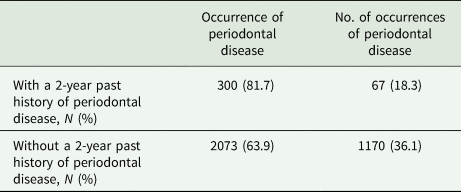
Table 3 presents the adjusted ORs associated with the demographics, clinical characteristics and prescriptions for the risk of treated periodontal disease in all patients, as determined using logistic regression. Female sex (adjusted OR 1.40; 95% confidence interval [CI] 1.20–1.63; p < 0.001), younger age (adjusted OR 1.02; p < 0.001), 2-year history of periodontal disease (adjusted OR 2.45; 95% CI 1.84–3.26; p < 0.001) and high income level (adjusted OR 2.24; 95% CI 1.64–3.06; p < 0.001) were independent risk factors for periodontal disease. Compared with nonusers, the ORs of FGA, SGA, anticholinergic and antihypertensive users are 1.89 (95% CI 1.54–2.32; p < 0.001), 1.33 (95% CI 1.14–1.58; p = 0.001), 1.24 (95% CI 1.03–1.50; p = 0.025) and 1.91 (95% CI 1.64–2.23; p < 0.001), respectively, after adjustment for sex, age, 2-year history of periodontal disease, income level, geographical region and the index date.
Table 3. Risk of periodontal disease in patients with newly diagnosed schizophrenia during 1-year follow-up

OR, odds ratio; CI, confidence interval.
a After adjustment for age, sex, income level, geographical region, 2-year history of periodontal disease, index date and concomitant prescriptions.
Furthermore, we analysed the adjusted OR for treated periodontal disease following potential hyposalivation or hypersalivation attributable to antipsychotic or other medication use (Table 4). FGA-induced hyposalivation was associated with an increased risk of treated periodontal disease (adjusted OR 2.00; 95% CI 1.63–2.46; p < 0.001). Similarly, anticholinergic- and antihypertensive-induced hyposalivation were associated with risks of treated periodontal disease (adjusted OR 1.27; 95% CI 1.05–1.53; p = 0.015 v. adjusted OR 1.90; 95% CI 1.63–2.22; p < 0.001, respectively). Hence, FGA-induced hypersalivation in patients with treated periodontal disease was identified as a protective factor (adjusted OR 0.72; 95% CI 0.59–0.88; p = 0.001).
Table 4. Adjusted ORs of periodontal disease in patients with newly diagnosed schizophrenia due to potential hyposalivation and hypersalivation caused by antipsychotics and other medications

OR, odds ratio; CI, confidence interval.
a After adjustment for age, sex, income level, geographical region, 2-year history of periodontal disease, index date and adverse effects of concomitant prescriptions.
Discussion
According to a review of the relevant literature, this is the first study assessing the early development of periodontal disease in patients with schizophrenia. Data of 3610 patients newly diagnosed with schizophrenia between 2000 and 2009 were analysed. During the 1-year follow-up period, approximately two-thirds of these patients underwent periodontal disease treatment. Young age; female sex; high-income level; 2-year history of periodontal disease and exposure to FGAs, SGAs, anticholinergics and antihypertensives were independent risk factors for periodontal disease in the patients newly diagnosed with schizophrenia. Moreover, FGA-, anticholinergic- and antihypertensive-induced hyposalivation were associated with an increased risk of periodontal disease. Hence, hypersalivation caused by FGAs was considered a protective factor.
Incidence of periodontal disease in schizophrenia
The incidence of periodontal disease in patients with schizophrenia varies with ethnicity, time, socioeconomic status, psychophysical condition, definition and length of illness and assessment tools (Angelillo et al., Reference Angelillo, Nobile, Pavia, De Fazio, Puca and Amati1995; Tang et al., Reference Tang, Sun, Unqvari and O'Donnell2004; Pihlstrom et al., Reference Pihlstrom, Michalowicz and Johnson2005; Arnaiz et al., Reference Arnaiz, Zumárraga, Díez-Altuna, Uriarte, Moro and Pérez-Ansorena2011; Gurbuz et al., Reference Gurbuz, Alatas, Kurt, Dogan and Issever2011; Teng et al., Reference Teng, Su, Chang and Lai2011). The following three studies – including the current study – among ethnic Chinese populations are considered as examples (Tang et al., Reference Tang, Sun, Unqvari and O'Donnell2004; Teng et al., Reference Teng, Su, Chang and Lai2011). A cross-sectional survey of oral health in central Taiwan using the community periodontal index (CPI) showed that 90% of psychiatric inpatients (schizophrenia: 61%, length of illness: approximately 6 years) had poor periodontal health (Teng et al., Reference Teng, Su, Chang and Lai2011). A dental study in Hong Kong demonstrated that 98.5% of inpatients with chronic schizophrenia (length of illness: 20 years) had poor periodontal health, as assessed using the standardised dental evaluation of the World Health Organization (Tang et al., Reference Tang, Sun, Unqvari and O'Donnell2004). In the current study, the incidence of periodontal disease in patients with newly diagnosed schizophrenia (length of illness: 1 year or less) in Taiwan was 65.7%, as assessed from dentist visit records. Although time, duration of schizophrenia, assessment tools, psychophysical condition and socioeconomic status were different in all three studies, the data crudely illustrated that in terms of duration of schizophrenia, the incidence of periodontal disease in patients with schizophrenia increases from 65.7% after approximately 1 year to 98.5% after 20 years from the schizophrenia diagnosis. The results possibly imply that early preventive measures could prevent the incidence of periodontal disease in approximately one-third of patients newly diagnosed with schizophrenia.
Risk factors for periodontal disease in schizophrenia
Most evidence demonstrates that old age (Angelillo et al., Reference Angelillo, Nobile, Pavia, De Fazio, Puca and Amati1995; Tang et al., Reference Tang, Sun, Unqvari and O'Donnell2004; Arnaiz et al., Reference Arnaiz, Zumárraga, Díez-Altuna, Uriarte, Moro and Pérez-Ansorena2011; Gurbuz et al., Reference Gurbuz, Alatas, Kurt, Dogan and Issever2011; Teng et al., Reference Teng, Su, Chang and Lai2011) and male sex (Gurbuz et al., Reference Gurbuz, Alatas, Kurt, Dogan and Issever2011; Gopalakrishnapillai et al., Reference Gopalakrishnapillai, Iyer and Kalantharakath2012) are associated with significantly higher risks of periodontal disease in patients with schizophrenia. This evidence was obtained from patients with chronic schizophrenia and periodontal disease by using assessment tools such as the CPI. Therefore, we assessed the presence of periodontal disease by identifying dentist visits by patients within 1 year of a schizophrenia diagnosis. Differences between subjects and assessment tools may have led to the identification of young age and female sex as risk factors for treated periodontal disease in our study. In other words, young female patients in the early stage of schizophrenia may have an opportunity or good insights for treatment of periodontal disease with good prognosis. On the contrary, elderly male patients with chronic schizophrenia had poor periodontal health. The findings thus suggest that primary care staff should be more concerned about early prevention of periodontal disease in elderly male patients with schizophrenia because this population may not visit a dentist frequently and their periodontal disease could worsen when they enter the chronic stage of schizophrenia.
Low income level is a known crucial risk factor for periodontal disease in the general population (Pihlstrom et al., Reference Pihlstrom, Michalowicz and Johnson2005). However, high income level was a risk factor for treated periodontal disease in patients with newly diagnosed schizophrenia – probably because the patients belonged to a high income level class in this study were well aware that dental visits are necessary for maintaining good oral hygiene. The financial burden of dental expenses was limited for the patients in our study because Taiwan's National Health Institutes provide most Taiwanese with basic dental care without large copayments (Teng et al., Reference Teng, Su, Chang and Lai2011). Notably, 2-year history of periodontal disease was the most vital risk factor for periodontal disease in the current study. Therefore, the incidence of periodontal disease increased dramatically from 9% at 2 years before schizophrenia diagnosis to 59.5% in the 1 year after. This increase might be ascribed to the relief that psychiatric treatment can provide to a patient – increasing in a patient's sense of reality and willingness to seek treatment for periodontal disease, leading to more than 6-fold dental visits – albeit with the adverse effects of antipsychotics.
Antipsychotics and periodontal disease in schizophrenia
Little evidence is available about the adverse effects of antipsychotics and periodontal disease in patients with schizophrenia because most previous surveys had small-to-medium samples, and complex patterns of antipsychotics were prescribed to the study subjects (Hede, Reference Hede1995; Gurbuz et al., Reference Gurbuz, Alatas, Kurt, Dogan and Issever2011; Teng et al., Reference Teng, Su, Chang and Lai2011; Gopalakrishnapillai et al., Reference Gopalakrishnapillai, Iyer and Kalantharakath2012; Eltas et al., Reference Eltas, Kartalcı, Eltas, Dündar and Uslu2013). The current paper is among the few reports (Hede, Reference Hede1995; Gopalakrishnapillai et al., Reference Gopalakrishnapillai, Iyer and Kalantharakath2012) that investigate the effects of antipsychotics on periodontal disease in patients with schizophrenia. It was impossible to assess the effect of each antipsychotic; we classified all antipsychotics and other medications into four categories: FGAs, SGAs, anticholinergics and antihypertensives. The findings of the current study illustrated that all four types of medication could accelerate development of periodontal disease, and the order of high to low risk is as follows: antihypertensives, FGAs, SGAs and anticholinergics. Extrapyramidal symptoms often occur with antipsychotics, particularly FGAs, so physicians prescribe co-medications such as anticholinergics or antihypertensives to alleviate these symptoms (Teng et al., Reference Teng, Su, Chang and Lai2011; Gopalakrishnapillai et al., Reference Gopalakrishnapillai, Iyer and Kalantharakath2012; Hu et al., Reference Hu, Chou, Wen, Hsieh, Tsai, Yang, Yang and Lin2016). Unfortunately, anticholinergics and antihypertensives can exacerbate the resultant periodontal disease. Therefore, clinicians should prescribe antipsychotics and other medications to the least extent possible under efficacious pharmacotherapy for schizophrenia.
Several reports have revealed that hyposalivation (xerostomia) may increase the risk of periodontal disease (Wagaiyu and Ashley, Reference Wagaiyu and Ashley1991; Hirotomi et al., Reference Hirotomi, Yoshihara, Ogawa, Ito, Igarashi and Miyazaki2006; Eltas et al., Reference Eltas, Kartalcı, Eltas, Dündar and Uslu2013; Mizutani et al., Reference Mizutani, Ekuni, Tomofuji, Azuma, Kataoka, Yamane, Iwasaki and Morita2015); however, only one of these reports focused on patients with schizophrenia, the findings of which suggested that patients with schizophrenia who had reduced salivary flow rate (hyposalivation) due to antipsychotics were at higher risk of periodontal disease than those with increased salivary flow rate (hypersalivation) (Eltas et al., Reference Eltas, Kartalcı, Eltas, Dündar and Uslu2013) – a result similar to our study. We used the incidence of periodontal disease associated with antipsychotics and other medications as a surrogate because detailed data on the actual saliva flow rate of the patients with schizophrenia who were taking these medications were not available from the NHIRD. The benefit of using periodontal disease as a surrogate is switched to requiring medical attention. Our findings demonstrated that hyposalivation induced by FGA, anticholinergic and antihypertensive is potentially associated with increased risk of periodontal disease; hence, FGA-induced hypersalivation in periodontal disease is considered a protective factor. Although our statistical analyses could not provide sufficient information on the linked pharmacopathology between the adverse effects of saliva and periodontal disease, the data demonstrate how clinicians can reduce periodontal disease caused by the adverse effects of antipsychotics during pharmacotherapy for schizophrenia. The findings suggest that clinicians should avoid iatrogenic adverse effects on saliva while prescribing antipsychotics as far as possible and actively manage the adverse effects and ensure early dental referral. The possible underlying causal pharmacological mechanism must be determined in future studies.
Strengths and limitations of the study
The major strength of the current study was the use of a large population-based cohort that enabled us to evaluate the relationship between antipsychotics and risk factors for periodontal disease in the early stages of schizophrenia. The findings may prove favourable to prevent the development of periodontal disease in patients with schizophrenia, especially the ethnic Chinese population. The well-determined temporal relationship between antipsychotic prescription and the occurrence of periodontal disease was another strength of this study. We obtained not only rigorous illness diagnoses but also correct medication information from the NHIRD. The study sample was identified based on the association between an ambulatory care expenditures database (ICD-9-CM codes and psychiatrists) and the prescription claims database (pharmacotherapy for schizophrenia) to increase the diagnostic accuracy of schizophrenia. We also considered significant covariates, including underlying diseases such as HIV/AIDS, diabetes mellitus, osteoporosis, chronic pulmonary diseases and a history of periodontal disease.
This study had several limitations. First, diagnoses of both periodontal disease and schizophrenia could have been underestimated, for instance, because of Berkson's bias, in which hospital cases and controls in a case–control study can be systematically different from one another because the combination of exposure to risk and disease occurrence increases the likelihood of admission. Hence, we surveyed treated patients with schizophrenia as well as the possible consequences of periodontal disease. Accordingly, treatment-naïve patients may have had only a limited effect on our analysis. Second, patient adherence to medications could not be evaluated because of the prescription claims database in this study. However, medication nonadherence would most likely have resulted in a nondifferentiated misclassification of exposure leading to possible underestimation of actual risk. Third, only treated periodontal disease indicated by the patients’ medical records was considered as the measure of periodontal disease occurrence in this study as opposed to all cases of periodontal disease wherein patients with schizophrenia developed periodontal disease but did not visit a dentist. Fourth, nonavailability of information on dietary, lifestyle and other potential risk factors for periodontal disease, such as illness severity, biochemistry data and patients’ unhealthy lifestyles such as tobacco consumption and poor oral hygiene (Albandar, Reference Albandar2002; Ramon et al., Reference Ramon, Grinshpoon, Zusman and Weizman2003; Scully and Bagan, Reference Scully and Bagan2004; Pihlstrom et al., Reference Pihlstrom, Michalowicz and Johnson2005; Dumitrescu et al., Reference Dumitrescu, Dogaru and Dogaru2008; Chu et al., Reference Chu, Yang, Chou, Chiu and Chi2012; Kossioni et al., Reference Kossioni, Kossionis and Polychronopoulou2012; Thomson et al., Reference Thomson, Sheiham and Spencer2012; Genco and Borgnakke, Reference Genco and Borgnakke2013; Morales-Chávez et al., Reference Morales-Chávez, Rueda-Delgado and Peña-Orozco2014; Hu et al., Reference Hu, Chou, Wen, Hsieh, Tsai, Yang, Yang and Lin2016) – was considered.
Conclusions
In this paper, we highlight early prevention of periodontal disease in patients with schizophrenia. Based on our findings and previously reported evidence, we suggest that more care should be provided to men with schizophrenia who have a history of periodontal disease so as to prevent further periodontal degeneration. We also emphasise that in addition to paying more attention to development of periodontal disease, assessing oral health regularly, assisting with oral hygiene and lowering consumption of sugary drinks and tobacco, physicians should prescribe antipsychotics to the least extent possible and avoid iatrogenic adverse effects on saliva as far as possible under efficacious pharmacotherapy for schizophrenia.
Acknowledgement
All authors acknowledge the Taiwan NHRI and BNHI for the data. The interpretation and conclusions described in this paper do not represent those of the NHRI and BNHI.
Author contributions
J.-H. Tsai and K.-F. Hu designed the study and wrote the protocol. J.-H. Tsai, Y.-H. Chou, P.-S. Ho, C.-H.R. Lin and H.-Y. Chuang interviewed and assessed the database. J.-H. Tsai, K.-F. Hu and P.-S. Ho managed the literature, analysed and wrote the manuscript and tables. P.-S. Ho, C.-H.R. Lin and H.-Y. Chuang analysed the data statistically. All authors have contributed to and have approved the final manuscript.
Financial support
None.
Conflict of interest
None.
Ethical standards
This study was approved by the Institutional Review Board at Kaohsiung Medical University Hospital (KMUH-IRB-EXEMPT-20140030) and informed consent was waived because of the use of previously stored de-identified medical information from the NHIRD.
Availability of data and materials
In the current study, there are ethical or legal restrictions on sharing a de-identified data set. Therefore, we have provided contact information for a data access committee, see NHRID_SQL Generator http://sqlgen.net.nsysu.edu.tw/SQL_Generator/General_Searching.html (in English, cited on 2019/2/13).
Appendix A
Table A1. ICD-9-CM codes and Anatomical Therapeutic Chemical [ATC] classification system codes used in this study

Table A2. Hyposalivation and hypersalivation caused by antipsychotics and other medications









