Introduction
Electroconvulsive therapy (ECT) has been used in the treatment of major mental illness, particularly depression, since its initial recorded use in 1938 (Cerletti and Bini, Reference Cerletti and Bini1938). Since then, significant improvements have emerged in its evidence-based practice (Kirov et al., Reference Kirov, Jauhar, Sienaert, Kellner and McLoughlin2021).
ECT has been evaluated and approved by the National Institute for Health and Care Excellence (NICE) in the UK (NICE, 2003), Food and Drug Administration (FDA) in the USA (FDA, 2018) and various international professional organisations (Bennabi et al., Reference Bennabi, Charpeaud, Yrondi, Genty, Destouches, Lancrenon, Alaïli, Bellivier, Bougerol, Camus and Dorey2019; Malhi et al., Reference Malhi, Bell, Bassett, Boyce, Bryant, Hazell, Hopwood, Lyndon, Mulder, Porter and Singh2020). Both NICE and FDA additionally incorporated specialist/professional and patient/carer group perspectives. Specifically, NICE used a systematic review commissioned by the Department of Health (Greenhalgh et al., Reference Greenhalgh, Knight, Hind, Beverley and Walters2005), based on the systematic review and meta-analysis by the UK ECT Group (UK ECT Review Group, 2003).
Despite this, misgivings and misunderstandings persist about both relative safety and efficacy of ECT. For example, a group of mental health professionals, patients and relatives recently wrote to the Chair of the Care Quality Commission (CQC) in the UK, stating: ‘Given the high risk of permanent memory loss and the small mortality risk, the longstanding failure to determine whether or not ECT works means that its use should be immediately suspended until a series of well designed, randomised, placebo controlled studies have investigated whether there really are any significant benefits against which the proven significant risks can be weighed’ (Read, Reference Read2020). Calls have also been made for an independent commission into ECT in the UK (Cunliffe and Johnstone, Reference Cunliffe and Johnstone2020).
Evidence cited to underpin these claims consists of a narrative review of the efficacy of ECT in depression (Read and Bentall, Reference Read and Bentall2010) published in this journal, and a recent narrative review, which aimed to assess the quality of randomised controlled trials (RCTs) of ECT v. simulated ECT (sECT) for treatment of depression (Read et al., Reference Read, Kirsch and McGrath2019). Although examining data from the same time period (no new placebo-controlled studies have been published in the interim), conclusions of both reviews oppose the findings and recommendations from NICE, FDA and multiple meta-analyses (Janicak et al., Reference Janicak, Davis, Gibbons, Ericksen, Chang and Gallagher1985; Kho et al., Reference Kho, van Vreeswijk, Simpson and Zwinderman2003; UK ECT Review Group, 2003; Pagnin et al., Reference Pagnin, de Queiroz, Pini and Cassano2004; Greenhalgh et al., Reference Greenhalgh, Knight, Hind, Beverley and Walters2005; Gábor and László, Reference Gábor and László2005). Given this discrepancy and that these two narrative reviews provide the basis for recent clamour against ECT, we sought to examine these reviews.
What is the evidence base for efficacy of ECT in the treatment of depression?
Briefly, clinical trial evidence for ECT in depression can be divided into original placebo-controlled (sECT) RCTs, further RCTs assessing ECT v. antidepressant, ECT v. repetitive transcranial magnetic stimulation (rTMS), and RCTs comparing different forms of ECT and pairwise and network meta-analyses of these trials.
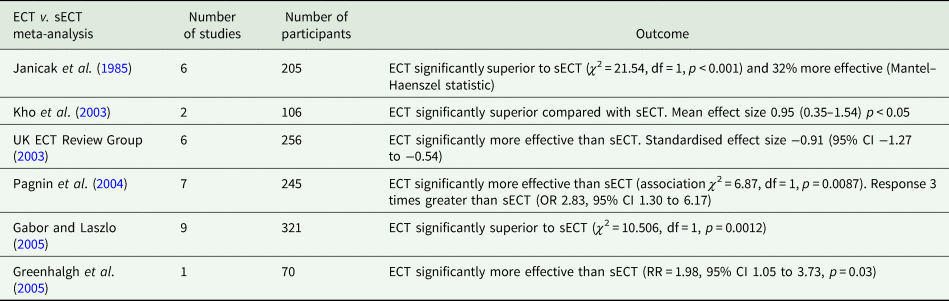
Ten ECT-sECT RCTs for depression have been published (summarised in Table 1). In a systematic review and meta-analysis, the UK ECT Review Group (UK ECT Review Group, 2003) identified six relevant trials, demonstrating ECT was more effective than sECT with a large standardised effect size of −0.91 (95% CI −1.27 to −0.54). These findings are broadly consistent across relevant meta-analyses (summarised in Table 2). The UK ECT Review Group also found ECT superior to antidepressants for depression (effect size −0.80 [95% CI −1.29 to −0.29]).
Table 1. Original ECT v. sECT trials identified by Read and Bentall (Reference Read and Bentall2010)
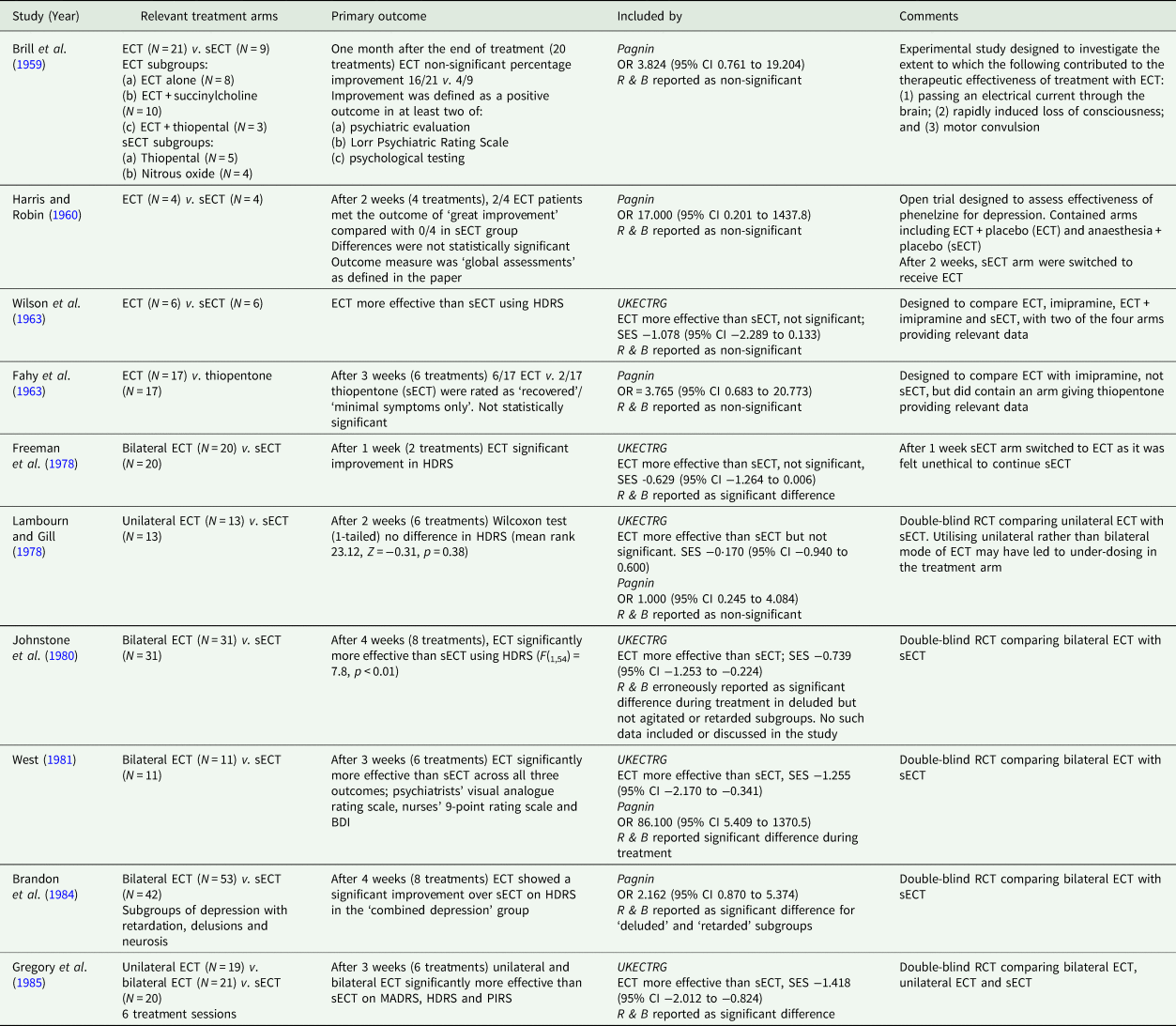
BDI, Beck Depression Inventory; HDRS, Hamilton Depression Rating Scale; MADRS, Montgomery Åsberg Depression Rating Scale; MPRS, Malamud Psychiatric Rating Scale; PIRS, Psychological Impairments Rating Scale; CI, confidence interval; SES, standardised effect size.
Pagnin, Pagnin et al. (Reference Pagnin, de Queiroz, Pini and Cassano2004); R & B, Read and Bentall (Reference Read and Bentall2010); UKECTRG, UK ECT Review Group (2003).
Table 2. Meta-analyses of ECT v. sECT studies
The original ECT-sECT RCTs, conducted between the 1950s and mid-1980s, would not meet contemporary standards of evidence-based medicine (EBM) (as is the case for the majority of medical trials from that era). Numerous additional modern RCTs have assessed the efficacy of ECT against non-pharmacological interventions such as rTMS. In a systematic review and meta-analysis comparing ECT with rTMS (n = 294), remission rates were significantly higher for ECT v. rTMS (52 v. 34% respectively, OR = 0.46 [95% CI: 0.22 to 0.96; z = −2.06; p =0.04]) with a significantly more pronounced reduction of depressive symptoms in the ECT group (Hedges' g = −0.93; p = 0.007) (Berlim et al., Reference Berlim, Van den Eynde and Daskalakis2013). A subsequent meta-analysis (n = 429) (Ren et al., Reference Ren, Li, Palaniyappan, Liu, Wang, Li and Rossini2014) found ECT superior to high-frequency rTMS in response (64.4 v. 48.7%, RR = 1.41, p = 0.03) and remission (52.9 v. 33.6%, RR = 1.38, p = 0.006). Neither review found a significant difference in discontinuation between groups. Whilst this illustrates improved trial design (contemporary ECT modes and active comparators), these trials usually lack blinding of participants.
Andrade (Andrade, Reference Andrade2021) gives an example of three RCTs of ECT v. active comparators (Sackeim et al., Reference Sackeim, Decina, Kanzler, Kerr and Malitz1987, Reference Sackeim, Prudic, Devanand, Kiersky, Fitzsimons, Moody, McElhiney, Coleman and Settembrino1993, Reference Sackeim, Prudic, Devanand, Nobler, Lisanby, Peyser, Fitzsimons, Moody and Clark2000) that demonstrate the efficacy of bilateral ECT and high-dose right unilateral ECT in people with Major Depressive Disorder (MDD) compared to low-dose right unilateral ECT. Meta-analytic data also demonstrate the efficacy of brief-pulse right unilateral ECT compared to ultra-brief pulse right unilateral ECT (standardised mean difference = 0.25 [95% CI 0.08 to 0.41]; p = 0.004) (Tor et al., Reference Tor, Bautovich, Wang, Martin, Harvey and Loo2015). As Andrade (Reference Andrade2021) points out, high-quality RCTs, utilising contemporary ECT techniques demonstrate the efficacy of ECT when tested blindly against an active comparator (in the form of an alternative mode of ECT).
Thus, the evidence base for ECT consists of RCTs providing converging evidence consistently demonstrating greater efficacy than numerous comparators, including sECT, pharmacological and non-pharmacological interventions as well as alternative modes of ECT.
Read and Bentall (2010)
In their narrative review, Read and Bentall identified ten studies from 1959 to 1985 comparing ECT with sECT and eight meta-analyses for ECT compared with sECT, in depression. In addition to summarising these studies, they covered potential side-effects, including what they describe as ‘brain damage’. They report ‘…placebo-controlled studies show minimal support for effectiveness with either depression or “schizophrenia”…’ and conclude, ‘Given the strong evidence (summarised here) of persistent and, for some, permanent brain dysfunction, primarily evidenced in the form of retrograde and anterograde amnesia, and the evidence of a slight but significant increased risk of death, the cost-benefit analysis for ECT is so poor that its use cannot be scientifically justified.’
These bold claims directly contradict evidence available at the time, as well as recently published reviews (Mutz et al., Reference Mutz, Vipulananthan, Carter, Hurlemann, Fu and Young2019; Andrade, Reference Andrade2021; Kirov et al., Reference Kirov, Jauhar, Sienaert, Kellner and McLoughlin2021). Examining this review from an evidence-based perspective highlights common problems in narrative as opposed to systematic reviews.
Lack of an effect size measure, i.e. meta-analysis
RCTs included in the Read and Bentall review are summarised in Table 1, which also indicates inclusion in either of the two major meta-analyses available before 2010 from the UK ECT Review Group (UK ECT Review Group, 2003) and Pagnin et al. (Pagnin et al., Reference Pagnin, de Queiroz, Pini and Cassano2004). A number of the historical studies comparing ECT to sECT were methodologically flawed and, therefore, only six of the ten were included in the comprehensive systematic review by the UK ECT Group. No clear study inclusion criteria are provided in Read and Bentall's narrative review.
Table 1 (p. 336) states only five of ten studies were statistically significant. Such vote-counting is a flawed method of research synthesis, and acknowledged as misleading (see Hedges and Olkin, Reference Hedges and Olkin1980; Gurevitch et al., Reference Gurevitch, Koricheva, Nakagawa and Stewart2018) – not least of all because it does not quantify the magnitude of group differences or consider sample sizes. One study (Harris and Robin, Reference Harris and Robin1960) only included four patients in each arm (eight in total), another (Wilson et al., Reference Wilson, Vernon, Sandifer and Guin1963) only 6 patients (12 in total); therefore, statistical significance of the individual study does not necessarily convey useful information. In some of these studies (e.g. Brill et al., Reference Brill, Crumpton, Eiduson, Grayson, Hellman and Richards1959; Harris and Robin, Reference Harris and Robin1960), ECT showed greater efficacy than comparator, though not significantly so. When seven of the studies included in the review were meta-analysed by Pagnin et al (Pagnin et al., Reference Pagnin, de Queiroz, Pini and Cassano2004) six years earlier, a statistically significant superiority for ECT over sECT was seen (see Table 2). A related point is selective citing of follow-up data, and conclusions drawn, when studies were not conducted to test longer-term effects and had naturalistic designs following acute treatment, i.e. were not conducted under RCT conditions.
Given the lack of reporting of an effect size across all included trials, we conducted our own meta-analysis of trials included in the narrative review. Read and Bentall suggested Ulett et al. (Reference Ulett, Smith and Gleser1956) be excluded as the sample would not meet the criteria for depression diagnosis and we therefore excluded it. We found a pooled effect size that was both large and significant (g = −0.85 [95% CI −1.08 to −0.63]) (see Fig. 1). Heterogeneity was extremely low (I 2 = 0) and non-significant (Q = 8.96, df = 9, p = 0.44). This effect size is comparable to that reported by the UK ECT group, −0.91 (95% CI −1.27 to −0.54) derived from six trials.
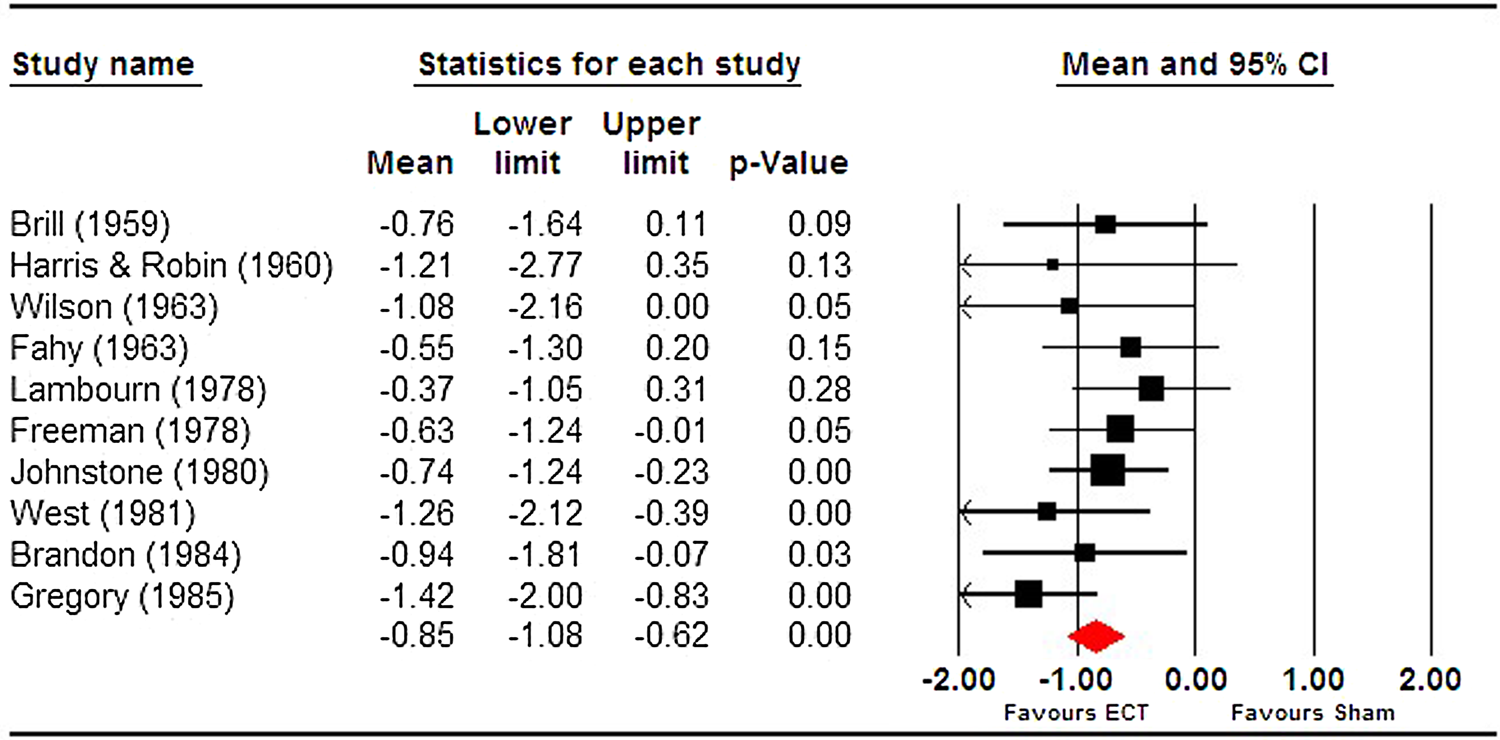
Fig. 1. Meta-analysis of ECT vs sECT trials for depression, included in Read and Bentall (Reference Read and Bentall2010).
Selective citing/reporting
In their discussion of ECT's effects on memory, Read and Bentall (Reference Read and Bentall2010) and Read et al. (Reference Read, Kirsch and McGrath2019) both rely on a single systematic review of patient perspectives on ECT (Rose et al., Reference Rose, Fleischmann, Wykes, Leese and Bindman2003) that included seven studies measuring subjective memory impairment as evidence of memory dysfunction. We note this review has been criticised (Bergsholm, Reference Bergsholm2012), on account of included studies. The review stated the inclusion of reports from at least 6 months post-ECT, but three of the seven studies failed to meet this criterion – obtaining patient self-report much sooner, e.g. Pettinati et al. (Reference Pettinati, Tamburello, Ruetsch and Kaplan1994) at <48 h after ECT. It should be noted that Rose et al. did not report control data, when it is known that self-reported memory problems also occur in sham ECT and some research shows that such problems might be indistinguishable from those in real ECT (Frith et al., Reference Frith, Stevens, Johnstone, Deakin, Lawler and Crow1983). Indeed, Frith et al. argue that ‘…complaints about the memory problems seem not to be associated with real ECT, but with a relatively high degree of depression and an unsuccessful treatment’ (p. 615), and cognitive impairment in depression is recognised in MDD (Rock et al., Reference Rock, Roiser, Riedel and Blackwell2014).
In contrast to Rose et al. in a 2019 systematic review (Jones and McCollum, Reference Jones and McCollum2019), out of seven subjective memory studies identified and conducted prior to the Read and Bentall (Reference Read and Bentall2010) review, four found improved subjective memory scores (Coleman et al., Reference Coleman, Sackeim, Prudic, Devanand, McElhiney and Moody1996; Ng et al., Reference Ng, Schweitzer, Alexopoulos, Celi, Wong, Tuckwell, Sergejew and Tiller2000; Schulze-Rauschenbach et al., Reference Schulze-Rauschenbach, Harms, Schlaepfer, Maier, Falkai and Wagner2005; Arts et al., Reference Arts, Peters, Ponds, Honig, Menheere and van Os2006), two reported no difference (Frith et al., Reference Frith, Stevens, Johnstone, Deakin, Lawler and Crow1983; Berman et al., Reference Berman, Prudic, Brakemeier, Olfson and Sackeim2008), only one showed reduced subjective memory scores during ECT, in a mixed sample of people with mania, schizophrenia and depression, noting normalisation at follow-up (Ikeji et al., Reference Ikeji, Ohaeri, Osahon and Agidee1999). While Read and Bentall (Reference Read and Bentall2010) selectively cite additional studies regarding retrograde and anterograde amnesia, they do not include the six subjective memory studies that demonstrated views contrary to their own, despite all being available at the time.
In a meta-analysis on objective cognitive performance associated with ECT reported in the same year and including 84 studies (75 of which included memory domains) (Semkovska and McLoughlin, Reference Semkovska and McLoughlin2010) found significant decreases in cognitive performance occurred 0–3 days post-ECT in 72% of 24 cognitive variables (effect sizes ranging from −1.10 [95% CI −1.53 to −0.67] to −0.21 [95% CI −0.40 to 0.01]). Fifteen days post-ECT there were no negative effect sizes and 57% of variables showed a positive effect size (improved cognition), ranging from 0.35 [95% CI 0.07 to 0.63] to 0.75 [95% CI 0.43 to 1.08]. Read and Bentall (Reference Read and Bentall2010) only included one of the 75 objective memory studies (Neylan et al., Reference Neylan, Canick, Hall, Reus, Sapolsky and Wolkowitz2001) despite being available at the time. Read and Bentall (Reference Read and Bentall2010) defend the use of subjective memory scores, but do not provide a representative summary of evidence underpinning such scores or methodological issues surrounding comparison between subjective and objective measures of cognitive performance in depression.
A related point is ‘cherry-picking’ of outcome measures. For example, the Northwick Park trial's primary outcome measure was the Hamilton Depression Rating Scale (HDRS); the study was powered based on this measure, which showed a statistically significant improved outcome with real ECT (Johnstone et al., Reference Johnstone, Lawler, Stevens, Deakin, Frith, McPherson and Crow1980). Rather than focusing on the primary outcome and this significant difference, the authors highlight secondary outcomes that failed to show statistical significance.
Utilising the widely used AMSTAR 2 tool (Shea et al., Reference Shea, Reeves, Wells, Thuku, Hamel, Moran, Moher, Tugwell, Welch, Kristjansson and Henry2017) to assess the quality of reviews, we found Read and Bentall (Reference Read and Bentall2010) scored ‘critically low’ in quality. Analyses were independently conducted by two authors (CM and KL: see online Supplementary Table 3). Inter-rater reliability was 93.75% agreement and Cohen's k = 0.82 which indicates ‘almost perfect agreement’ (Landis and Koch, Reference Landis and Koch1977).
Read, Kirsch and McGrath Review (2019)
Read et al. (Reference Read, Kirsch and McGrath2019) aimed to assess the quality of ECT v. sECT RCTs and related meta-analyses. Eleven original RCTs and five meta-analyses were identified. They used their own un-validated 24-point quality scale to evaluate trials and commented on linked meta-analyses.
They reported variation in number of original studies included across meta-analyses, ranging from 1 to 7 and ‘little attention’ paid to the limitations of the original studies. The mean ‘quality score’ of original RCTs was 12.3 out of 24. The authors dedicated a substantial portion of the review to side-effects.
As with Read and Bentall (Reference Read and Bentall2010), Read et al. (Reference Read, Kirsch and McGrath2019) contains major methodological shortcomings related to EBM. Specific concerns include:
The review was not systematic and ‘critically low’ in quality
Read et al. (Reference Read, Kirsch and McGrath2019) failed to pre-register a protocol for their review. An essential feature of any high-quality systematic review is publication of a pre-registered protocol that identifies main objectives, key design features and planned analyses, inclusion/exclusion criteria, and both promote transparency and reduce potential bias. Read et al. (Reference Read, Kirsch and McGrath2019) scored ‘critically low’ when assessed using the AMSTAR 2 tool (Shea et al., Reference Shea, Reeves, Wells, Thuku, Hamel, Moran, Moher, Tugwell, Welch, Kristjansson and Henry2017). Analyses were independently conducted by two authors (CM and KL). Inter-rater reliability was 100% agreement (perfect agreement) and Cohen's k = 1 (Landis and Koch, Reference Landis and Koch1977) (see online Supplementary Table 3). According to AMSTAR 2 guidance, reviews that receive a ‘critically low’ ratings (i.e. both Read and Bentall (Reference Read and Bentall2010) and Read et al. (Reference Read, Kirsch and McGrath2019) means that ‘The review has more than one critical flaw and should not be relied on to provide an accurate and comprehensive summary of the available studies’.
Use of an un-validated quality scale, biased in favour of inflating poor-quality ratings
The authors combined the aspects of the Cochrane Risk of Bias tool with their own measures to assess the quality of ECT-sECT RCTs. The use of any quality scale itself is problematic, emphasised in the original paper from which ‘risk of bias’ domains were taken (Higgins et al., Reference Higgins, Altman, Gøtzsche, Jüni, Moher, Oxman, Savović, Schulz, Weeks and Sterne2011). Cochrane advance seven principles for assessing risk of bias, the first of which states: ‘Do not use quality scales’. The inclusion by Read et al., (Reference Read, Kirsch and McGrath2019) of items within the scale such as ‘patient ratings’ and ‘suicide measure’ was weighted the same as randomisation and blinding. While the authors tried to account for this by including multiple items for domains such as randomisation, blinding and diagnosis, the result is a scale that allows for inconsistent, internally invalid scores. Read et al. (Reference Read, Kirsch and McGrath2019) claimed to employ risk of bias domains derived from the Cochrane RoB tool (randomisation, blinding, incomplete outcome data and selective reporting). These domains (from the older RoB tool that Cochrane initially allowed while piloting the newer RoB2 tool) are used in ways not advocated by Cochrane. In direct conflict with Cochrane guidance, Read et al. (Reference Read, Kirsch and McGrath2019) collapse the Cochrane ‘No’ and ‘Unclear’ categories into a bimodal distinction where ‘No’ meant either no evidence OR clear negative evidence. Cochrane advice is that ‘A judgment of unclear risk should also be made if what happened in the trial is known but the associated risk of bias is unknown’. This re-scaling artificially inflates ‘No’ responses. To take a specific example, for the item ‘Decliners described’ (i.e. any description of people who were approached, but declined to participate), multiple studies are negatively scored because they do not report on any decliners (e.g. West, Reference West1981; Lambourn and Gill, Reference Lambourn and Gill1978). Under Cochrane guidance, the appropriate response is ‘unclear’. The inclusion by Read et al. of a quality-of-life scale such as HoNOS is difficult to understand, given HoNOS was not developed until several years after the most recent ECT-sECT trial in the early 1990s (Wing et al., Reference Wing, Beevor, Curtis, Park, Hadden and Burns1998). The same would be true for requiring a ‘validated depression scale for some of the included studies, e.g., Hamilton, Montgomery, Beck’. Several studies were conducted prior to the development of any of these scales – the earliest to be developed would appear to be Hamilton (Reference Hamilton1960).
Similarly, for other items, the scoring and scaling introduces further biases against accurate reporting of quality. For example, pooling of age and gender into a single criterion is baffling – these two features are independent. The scaling used means failing on either one alone is a failing on both and therefore biases against fair quality assessment. The authors provide no cut-off scores for their scale and thus no criterion demonstrating what would constitute a good or poor quality trial. Regardless of the multiple issues outlined, reporting a mean score of 12.3 out of 24 is uninterpretable. We have already seen that Read et al. have used the Cochrane RoB scale against advice; however, Cochrane are also clear that ‘The use of scales (in which scores for multiple items are added up to produce a total) is discouraged’. The failure to employ any standardised assessment of quality critically undermines both any objectivity and ability to draw conclusions about quality – especially as many such scales exist that are standardised, reliable and widely employed. As noted decades ago, Greenland (Reference Greenland1994) referred to the practice of quality scoring as ‘Perhaps the most insidious form of subjectivity masquerading as objectivity’ (p. 295). Given the above limitations, we assessed risk of bias for original ECT-sECT RCTs using the Cochrane RoB2 tool (Sterne et al., Reference Sterne, Savović, Page, Elbers, Blencowe, Boutron, Cates, Cheng, Corbett, Eldridge and Emberson2019), see online Supplementary Fig. 2, Table 4 and Table 5.
Effect sizes and study quality
Meta-analyses of small underpowered trials can exaggerate effect sizes (Dechartres et al., Reference Dechartres2013). While some individual simulated v. real ECT RCTs were adequately powered (Johnstone et al., Reference Johnstone, Lawler, Stevens, Deakin, Frith, McPherson and Crow1980; Brandon et al., Reference Brandon, Cowley, McDonald, Neville, Palmer and Wellstood-Eason1984) and demonstrated a significant effect, several have small sample sizes. Nevertheless, we found little evidence of a small study effect in terms of publication bias – Egger's test (intercept −0.75, p = 0.59) and Begg and Mazumdar Kendall's τ (−0.24, p = 0.33) were non-significant. The funnel plot however showed some visual asymmetry and a trim and fill analysis suggested three potentially missing studies – adjusting the effect size downward from −0.85 to −0.68.
Importantly, meta-regression showed the Read et al. (Reference Read, Kirsch and McGrath2019) quality scores were not significantly related to effect sizes (Z = 1.31, p = 0.19) (see online Supplementary Fig. 3). This has implications for their general criticism of past meta-analyses, where they argue, ‘All five of the meta-analyses claim that ECT is effective for depression but, as we have seen, they are all of a poor standard, not least because none of them pay sufficient attention to the quality of the papers on which they base this claim.’ The current analysis suggests at least two possibilities: either study quality is not a key driver of effect size in sECT trials or the Read et al. assessment of study quality fails to capture relevant quality differences.
Blinding and the placebo effect
Read et al. (Reference Read, Kirsch and McGrath2019) question the integrity of RCTs being ‘double blind’ on the assumption that the involvement of patients who had previously received ECT renders them ‘unblinded’ ‘because they know that ECT is always followed by headaches and disorientation’ (p. 89). They conclude ‘by not excluding people who have previously had ECT all 11 studies exaggerated the difference between ECT and SECT in ECT's favor, and that none were truly blind studies’ (p. 97). As pointed out by Anderson (Anderson Reference Anderson2021), this is itself a weak argument a priori, since side-effects such as headache and temporary confusion are common in general anaesthesia, and moreover, the claim ‘…is contradicted by audit figures (Scottish ECT Accreditation Network 2019) showing the incidence of post-ECT headache to be about 30% and confusion about 20%’ (Anderson, Reference Anderson2021).
Despite this, we conducted sub-group analysis to assess whether effect size was affected by the experience of previous ECT. Read et al. (Reference Read, Kirsch and McGrath2019) identified five trials that included people who had received ECT previously (Brill et al., Reference Brill, Crumpton, Eiduson, Grayson, Hellman and Richards1959; Freeman et al., Reference Freeman, Basson and Crighton1978; Lambourn and Gill, Reference Lambourn and Gill1978; Johnstone et al., Reference Johnstone, Lawler, Stevens, Deakin, Frith, McPherson and Crow1980; Brandon et al., Reference Brandon, Cowley, McDonald, Neville, Palmer and Wellstood-Eason1984) and five where previous ECT was unascertained (Harris and Robin, Reference Harris and Robin1960; Fahy et al., Reference Fahy, Imlah and Harrington1963; Wilson et al., Reference Wilson, Vernon, Sandifer and Guin1963; West, Reference West1981; Gregory et al., Reference Gregory, Shawcross and Gill1985) – note Read et al. (Reference Read, Kirsch and McGrath2019) collapsed both groups suggesting ‘None of the participants had had ECT at any time prior to the study’. Contrary to Read et al., (Reference Read, Kirsch and McGrath2019), the pooled effect size was significantly larger (Q = 4.01, p < 0.05) in trials where previous ECT was unknown or unreported (−1.13 [95% CI −1.50 to −0.76]) rather than those reporting previous treatment with ECT (−0.67 [95% CI −0.97 to −0.37]) (see online Supplementary Fig. 3). Hence, trials where patients previously received ECT did not bias in favour of the ECT arm through patients being ‘unblinded’. It is also worth noting that significant differences demonstrated when comparing different modes of ECT in contemporary trials (Sackeim et al., Reference Sackeim, Decina, Kanzler, Kerr and Malitz1987, Reference Sackeim, Prudic, Devanand, Kiersky, Fitzsimons, Moody, McElhiney, Coleman and Settembrino1993, Reference Sackeim, Prudic, Devanand, Nobler, Lisanby, Peyser, Fitzsimons, Moody and Clark2000) would not be found if ECT was simply a placebo response.
Misunderstanding the nature of EBM, placebo and active comparators
What of the authors' claim that the quality of the sECT-ECT studies is ‘so poor’ that they have no utility? One issue relates to the difference between identifying study limitations, contrasted with demonstrating study quality is ‘so poor’ that it should be discarded. EBM has developed over the last 50 years, such that many, if not most, trials from this era (including sECT-ECT studies) would likely not be consistent with contemporary standards yet are still of high enough standard to contribute to the evidence base. Second, it represents a misunderstanding of principles of EBM that both Read and Bentall (Reference Read and Bentall2010) and Read et al. (Reference Read, Kirsch and McGrath2019) focus exclusively on ECT-sECT RCTs and their associated meta-analyses as the only relevant evidence. The authors fail to consider these studies within the wider context of other relevant evidence.
Read et al., (Reference Read, Kirsch and McGrath2019) fail to comment on evidence from studies that demonstrate ECT's superiority to pharmacotherapy (e.g. Pagnin et al., Reference Pagnin, de Queiroz, Pini and Cassano2004, UK ECT Review Group, 2003) and non-pharmacological biological treatments both directly (Ren et al., Reference Ren, Li, Palaniyappan, Liu, Wang, Li and Rossini2014) and indirectly (Mutz et al., Reference Mutz, Vipulananthan, Carter, Hurlemann, Fu and Young2019). Moreover, where available, active comparators, not placebo controls, are the best treatments to include in comparison arms, as these can produce real side-effects, thereby maximising any placebo or nocebo effect (Stafford et al., Reference Stafford, Wagner and Lavori2009). As pointed out by Andrade (Andrade, Reference Andrade2021), there are ‘large, well-designed, well-conducted, and well-analyzed modern era RCTs that show that bilateral and high dose right unilateral ECT are more effective than low dose right unilateral ECT, or that brief-pulse ECT is more effective than ultrabrief-pulse ECT’, with the latter providing an active comparator superior to that of placebo sECT. In other words, the ECT-sECT RCTs exist in the context of an extensive, robust and converging evidence base.
Conclusion
Recent calls for banning ECT are based on selective narrative reviews written by authors who suggest ECT does not have efficacy v. sECT and has significant effects on patient safety that merit a public enquiry. Examining their evidence, we have identified numerous substantial problems that stem from these narrative reviews having inherent biases and major methodological shortcomings. We would suggest those concerned with interpreting evidence continue to use conventional standardised methods of systematic review and meta-analysis where possible and that policy decisions must continue to be based on this level of evidence.
Whilst we cannot agree with most of Read et al.'s empirical arguments, moving beyond disagreement is crucial. We therefore advocate for modern trials to optimise ECT side-effect monitoring, and studies to elucidate the mechanism of action of one of the most effective treatments we have in Psychiatry.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S2045796021000731
Financial support
National Institute for Health Research Biomedical Research Centre at South London and Maudsley National Health Service Foundation Trust and King's College London (to AHY and SJ).
Conflict of interest
AHY paid lectures and advisory boards for the following companies with drugs used in affective and related disorders Astrazenaca, Eli Lilly, Janssen, Lundbeck, Sunovion, Servier, Livanova. No share holdings in pharmaceutical companies. Lead Investigator for Embolden Study (AZ), BCI Neuroplasticity Study and Aripiprazole Mania Study Investigator initiated studies from AZ, Eli Lilly, Lundbeck, Wyeth Grant funding (past and present): NIMH (USA); CIHR (Canada); NARSAD (USA); Stanley Medical Research Institute (USA); MRC (UK); Wellcome Trust (UK); Royal College of Physicians (Edin); BMA (UK); UBC-VGH Foundation (Canada); WEDC (Canada); CCS Depression Research Fund (Canada); MSFHR (Canada); NIHR (UK). Janssen (UK). DMM has received brain stimulation research grants from the NHS Health Technology Assessment Programme (UK), National Association for Research on Schizophrenia and Affective disorders (USA) and the Health Research Board (Ireland); and received speaker's honoraria from MECTA and Otsuka, and an honorarium from Janssen for participating in an esketamine advisory board meeting. SJ has received honoraria for talks given for Sunovian and KRL has received honoraria for talks he has given for Lundbeck. KRL and CFM have no conflict of interest.





