INTRODUCTION
Healthcare-associated infections (HAIs) are a major public health issue because they are associated with significant morbidity and mortality, as well as prolongation of the hospital stay and increase in the cost of care [Reference Kilgore1, Reference Sheng2]. Prevention of HAIs requires a systematic approach based on surveillance and intervention and it is generally accepted that incidence and prevalence studies are the most appropriate means of achieving this [Reference Gastmeier3, Reference Freeman and McGowan4]. The gold standard for HAI surveillance is a large, prospective, on-site, continuous and hospital-wide study, but few hospitals opt for this approach and most international agencies, including CDC, recommend targeted surveillance [Reference Llata5]. However, point-prevalence studies are a rapid and inexpensive tool to obtain an overall view of the dimensions of the problem and to increase staff awareness, and are an acceptable alternative to the more costly and time-consuming hospital-wide or targeted surveillance [Reference Gastmeier3, Reference Sartor6]. The effectiveness and positive impact of such an endeavour increases with repeated investigations [Reference French7].
Although efforts have been made, the linkage of infection control programmes in distinct Greek hospitals and the establishment of a national HAI surveillance network have not been achieved [Reference Gikas8].
The purpose of this study was to estimate the magnitude of HAIs in the region of Western Greece and to describe relationships with possible predisposing factors, with the goal of providing evidence to support improvement in infection control measures.
METHODS
All six general hospitals in Western Greece were included in two 1-day prevalence surveys, on 16 December 2005 and 10 February 2006. Western Greece is one of 13 regions of Greece, which is further divided into the prefectures of Aitoloakarnania, Achaia, and Elia, and covers an area of 11 350 km2 (8·6% of the total area of Greece). According to the 2001 census, the population of this region was 741 282 (7% of the country's total population). The participating hospitals were the two tertiary hospitals of Patras: the University Hospital of Rio with 750 beds and St Andrew's Hospital with 404 beds. The other hospitals were regional: Agrinio (150 beds), Aigio (85 beds), Mesologi (150 beds) and Pyrgos (138 beds). All hospitals had internal medicine wards, cardiology, general surgery, orthopaedics, gynaecology, and urology wards, whereas the two tertiary hospitals had intensive-care units (ICUs), coronary-care units, neurology, nephrology, ophthalmology, neonatal unit, neurosurgery, and ENT wards. A paediatric department was present in Rio, Mesologi, and Agrinio hospitals and a plastic surgery department in St Andrew's Hospital.
The Laboratory of Public Health at the University of Patras supplied the hospitals with questionnaires including demographical and clinical data and written instructions on the data collection procedure.
Data collection
The data were collected on the day of survey by an infection control team (ICT) in each ward, consisting of a physician and a registered nurse. The ICT was responsible for completing standardized forms by reviewing patients' charts on every ward. Data collected included the date of birth, sex, date, time and diagnosis at admission, date of HAI onset, infection type and site. Prior and present antibiotic use and immunodeficiency status including neutropenia or immunosuppressive treatment and other predisposing factors such as diabetes mellitus and obesity were also included in the form, as well as the presence of an indwelling urinary or intravascular catheter. Culture and other laboratory results were also recorded. The confirmation of the presence of a HAI was performed by on-site observation.
The total number of in-patients, and the number and site of HAI for the different medical specialities were recorded separately. The major HAIs surveyed were lower respiratory tract infections, urinary tract infections, surgical site infections, bloodstream infections, and soft tissue infections; other less common infections (e.g. gastrointestinal infections, meningitis), were also recorded.
Definitions
The CDC criteria and definitions of nosocomial surgical wound infection, in its modification of 1992, were used for defining infections [Reference Garner9, Reference Horan10]. HAI was defined as occurrence of infection at least 48 h after hospital admission without evidence that the infection was present or incubating at the time of admission. All patients readmitted within 7 days after discharge who presented at time of study with a documented infection were evaluated for a possible HAI; this had to be documented on the day of the survey. Surgical-site infections were documented as HAI within 30 days after the operation, or 1 year in the case of infections associated with insertion of a prosthetic device.
Statistical analysis
Following the completion of the study, the raw data were anonymized and SPSS software version 16.0 (SPSS Inc., USA) was used for data entry and descriptive analysis.
The prevalence ratio of HAI was the number of these infections recorded over the total number of patients studied, expressed as a percentage. Some patients were diagnosed with more than one HAI, and these were registered as a single patient incident for the prevalence study. Prevalence of HAI was calculated as the total number of infections divided by the total number of the study population and for cases as the number of patients with HAI divided by the total number of the study population.The length of hospital stay before infection was defined as the number of days between admission and the onset of infection. No limit in the time interval between HAI onset and the survey was set, as long as the infection was considered to be active (i.e. under care). Prior antibiotic use was defined as the use of antibiotics during the last month before HAI onset.
RESULTS
In the first 1-day prevalence study 1096 patients were hospitalized (Table 1). Twenty-five HAIs were recorded in 24 patients giving a regional point-prevalence for HAI of 2·2%. In the second survey, 1084 patients were hospitalized and 40 patients presented with 45 HAIs giving a point prevalence of 3·7% (Table 1); the average regional prevalence of all recorded HAIs was 2·9%. In total there were 64 patients who presented with 70 HAIs; 33 males and 31 females with a mean age of 64 years (range 0–88). The prevalence ranged from 0 to 6·8% between hospitals and from 0 to 22·7% between wards with the highest rates in ICUs, neonatal wards and coronary-care units (Table 2). The mean interval between admission and HAI onset was 12·6 (s.d.=28·1) days and most patients had been hospitalized for <2 weeks before HAI onset. However, one patient was recorded with 212 days of hospitalisation and after excluding this case, the mean interval was 9·5 (s.d.=12·5) days.
Table 1. Prevalence of healthcare-associated infections (HAIs) in Western Greece
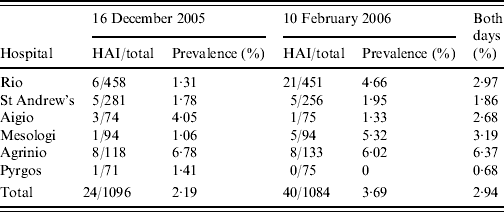
Table 2. Prevalence of healthcare-associated infections (HAIs) in the various wards of all hospitals
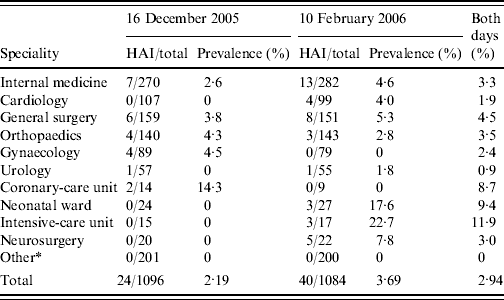
* Ophthalmology, Nephrology, Neurology, Paediatrics, Plastic surgery and ENT wards.
The mean duration from HAI onset to the survey day was 5·8 (s.d.=7·7) days. However, in almost 10% of the patients with an active HAI on the survey day, the onset of infection dated back >2 weeks. These were mostly critically ill patients with significant comorbidities and infected with multi-antibiotic resistance bacteria. In both surveys, HAIs were located most frequently in the urinary tract (34·2%), followed by lower respiratory tract (14·3%), bloodstream (14·3%), soft tissue (11·4%) and surgical sites (8·6%) (Table 3). Overall, 90% of patients with HAI had underlying diseases, such as cardiovascular disease, diabetes or cancer, or had indwelling vascular or urinary catheters (Table 3). Immunodeficiency was also due to chemotherapy, treatment with corticosteroids, renal failure or liver cirrhosis.
Table 3. Characteristics of healthcare-associated infections (HAIs) recorded in 64 patients with 70 HAIs
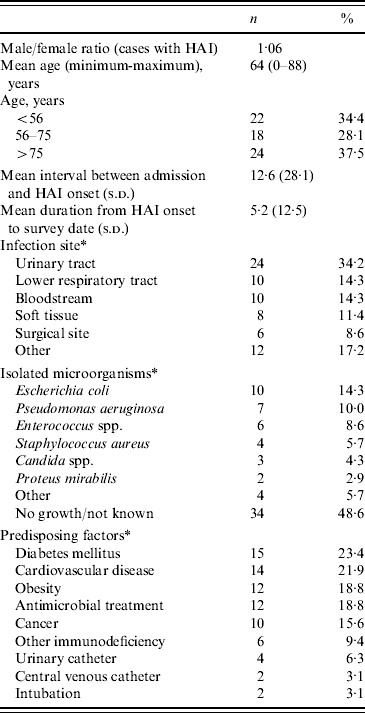
* More than one HAI, isolated microorganism or predisposing factor per patient.
Thirty-six of the 70 reported HAIs (51·4%) were confirmed microbiologically. The most frequently isolated microorganisms were Escherichia coli (14·3%), Pseudomonas aeruginosa (10%), Enterococcus spp. (8·6%) and Staphylococcus aureus (5·7%); Candida spp. and Proteus mirabilis were infrequent. Staphylococcus epidermidis, Klebsiella pneumoniae, Blastocladiomycota spp., Acinetobacter spp., Serattia spp. and Streptococcus type A accounted for 5·7% of infections. E. coli (41·6%) and enterococci (12·5%) predominated in the urinary tract while in pneumonia, P. aeruginosa (20%) and S. aureus (10%) were the most frequent isolates. P. aeruginosa caused half of all surgical-site infections and one-quarter of soft-tissue infections; S. aureus accounted for 12·5% of the latter infections.
The majority (70·3%) of the infected patients were receiving antibiotic therapy at the day of the study; 46·6% were receiving two antibiotics. The most frequently used antimicrobials were penicillins (26·6%), cephalosporins (24·4%), quinolones (20%) and carbapenems (20%).
We also found a tendency of patients aged >56 years to acquire infections earlier than younger people, although this difference was not statistically significant (P=0·124). Furthermore, urinary tract infections and lower respiratory tract infections tended to appear earlier than surgical-site and bloodstream infections (P=0·077).
DISCUSSION
The present study, through a transversal (1-day point prevalence) analysis, revealed the profile of HAIs in the wards of a tertiary university hospital and five district hospitals in Western Greece. The overall prevalence of HAIs in this region is similar to that reported from neighbouring countries, such as Italy (4·5%) and Turkey (2·5%) [Reference Brusaferro11, Reference Durmaz12]. However, a more recent survey of HAIs in 25 hospitals in Italy reported prevalence ranging from 0 to 24·4% with an overall prevalence of 8·9% [Reference Durando13], possibly reflecting the larger proportion of patients hospitalized in tertiary hospitals with HAI. Similarly, a study in a teaching hospital in Kosova reported a HAI prevalence of 17·4% which is also higher than other international rates [Reference Raka14].
In Northern European countries, the prevalence of HAI is often higher than in Mediterranean countries for reasons that remain unclear [Reference Hopmans15–Reference Gordts17], but higher levels of surveillance and monitoring by national networks, along with the use of more intensive antimicrobial therapy leading to increased antibiotic resistance may be contributory factors [Reference Struwe18]. In contrast very low prevalence rates reported from some developing countries may be attributed to underestimation, due to lack of facilities and support for HAI programmes [Reference Ider19].
In a study which was conducted in Greece in 2002, the observed prevalence of HAI was 9·6%, considerably higher than the rates found here [Reference Garner9, Reference Gikas20]. A possible explanation for this difference is that five university hospitals and nine other hospitals, mostly tertiary, participated in the early survey and tertiary and teaching hospitals, as shown in previously published studies [Reference Rüden21], often have higher infection rates than smaller and regional hospitals.
Our study is the first in Greece to include all general hospitals in a particular region (Western Greece). Unexpectedly, we did not observe major differences in the infection rates between smaller and larger hospitals, since the regional hospital of Agrinio had the highest prevalence and the tertiary hospital of St Andrew's had a relatively low prevalence (P=0·03). We believe that in Agrinio hospital the estimation of HAI was more complete due to the more experienced team that was responsible for recording and ascertaining patients with HAI, compared to the other hospital teams. Another explanation might be in the patients' profile; since a higher frequency of the elderly, stroke and comorbidities, were evident in HAI patients compared to the other hospitals. The University Hospital of Rio showed a low infection rate in the first survey and a higher rate in the second. The increased workload and the suboptimal level of training of nursing staff, as well as understaffing could be responsible not only for more adverse outcomes in patients, but also for the higher degree of under-diagnosis of infections in certain wards/floors [Reference Needleman22]. Comparing the infection rates between different departments of the hospitals, we found that ICUs and coronary-care units and neonatal wards had the highest infection rates, followed by the general surgery department. These results are consistent with data from other countries [Reference Gordts17, Reference Vincent23, Reference Eggimann and Pittet24]. The observed infection rate per site is also within the reported range of other studies [Reference Durando13, Reference Gravel25, Reference Gastmeier26]. In a multi-centre Greek study [Reference Kofteridis27], lower respiratory tract infections were the most prevalent HAI and partially associated with the high prevalence of these infections in ICUs, while a relatively small proportion of ICU and surgical patients determined the specific pattern of HAI site in our survey.
One of the limitations of our study was its cross-sectional design. This could account for the low proportion of patients with urinary catheters at the time of the survey, despite the observed high rate of urinary tract infections. In the literature, there is a very strong correlation between these two parameters [Reference Richards28, Reference Leone29]. Furthermore, we did not note the reasons for hospitalization for every patient, thus only a rough estimation of the prevalence of surgical-site infections in the appropriate wards could be achieved. However, it is assumed that <20% of overestimation took place, since 80% of the patients hospitalized in these wards underwent surgery.
The study shows additional interesting findings. Apart from the well established observation that elderly people are more susceptible to HAI [Reference Ellidokuz30, Reference Mintjes-de Groot31], mostly due to comorbidities, we found a tendency for patients aged >56 years to acquire infections earlier than younger people, although this difference did not reach statistical significance. This was mainly evident in urinary tract infections and was probably due to greater use of urinary catheters in the elderly, increased faecal incontinence, sedation and comorbidities, e.g. diabetes mellitus, which are associated with increased susceptibility to urinary tract infections [Reference Wagenlehner32–Reference Tsuchida34]. Generally, urinary tract and lower respiratory tract infections tend to appear earlier than surgical-site and bloodstream infections for reasons not well established, but in the elderly this may reflect an increased susceptible population.
In summary, our study provides important information on the prevalence of HAI in a relatively large region of Greece. The findings are consistent with the literature in respect of established predisposing factors for HAI and highlight the need for repeated point-prevalence studies in order to enhance surveillance techniques and improve infection control programmes. The data can be used for political and administrative purposes, aimed at a general effort for infection control in hospitals.
ACKNOWLEDGEMENTS
The authors thank the infection control teams for collecting the necessary data for the survey, as well as the Regional Health Authority, the Directors of the wards, and the Committees for Infectious Diseases for their support.
DECLARATION OF INTEREST
None.





