West Nile virus (WNV) is an emerging arbovirus with a zoonotic life-cycle [Reference Zeller and Schuffenecker1]. Virus transmission between birds (the virus reservoirs) requires the bite of an infected mosquito, although other transmission routes including oral transmission have been demonstrated experimentally [Reference Komar2, Reference Pérez-Ramírez, Llorente and Jiménez-Clavero3]. WNV has a complex eco-epidemiology that involves a wide range of vectors and great host diversity and is considered to be the most geographically widespread of all mosquito-borne flaviviruses [Reference Weissenböck4]. In humans and horses, both incidental hosts of the virus, WNV infections rarely result in clinical disease but can occasionally cause outbreaks that seriously affect animal and public health [Reference Beck5]. In humans, 80% of infections are asymptomatic, the remaining 20% being associated with influenza-like symptoms; despite this, in a few cases (<1%) the disease may appear as aseptic meningitis or encephalitis. It is important to note that these proportions vary according to the viral strain involved [Reference Sejvar6].
In the New World, the spread of WNV has had marked consequences and has resulted in the death of millions of birds since 1999 [Reference LaDeau, Kilpatrick and Marra7]. European birds infected with WNV rarely develop clinical symptoms and avian mortality is only reported infrequently in the wild [Reference Hubálek and Halouzka8]. Nevertheless, recent changes in the virus epidemiology suggest that an increase in its virulence has occurred [Reference Gray and Webb9]. Additionally, experimental infections in the laboratory have confirmed the pathogenic effect of many European WNV strains in birds from the Old World [Reference Pérez-Ramírez, Llorente and Jiménez-Clavero3, Reference Del Amo10], which highlights the importance of this virus in both public health and biological conservation [Reference Höfle11].
In Spain, in addition to the arrival of trans-Saharan migrant birds that are potentially exposed to WNV during their stay in Africa [Reference López12], local transmission events are thought to have occurred since the 1960s [Reference Hubálek and Halouzka8]. Conclusive evidence of WNV circulation in Spain came in the early 2000s when many bird species were detected with WNV antibodies [Reference Figuerola13] and the virus was identified in mosquitoes [Reference Vázquez14].
We analysed the presence of WNV antibodies in different migrant and resident species captured during 2013 as a part of an extensive study on WNV transmission in southern Spain. WNV and Usutu virus (USUV) belong to the same serogroup (Japanese encephalitis group; family: Flaviviridae) and a cross-reaction between these viruses may occur [Reference Llorente15]. As is the case for WNV, USUV actively circulates in southern Spain [Reference Vázquez14, Reference Höfle16]. Therefore, we confirmed our results by comparative neutralization tests using WNV and USUV in parallel. USUV, an African vector-borne flavivirus, has been recorded in recent years in a number of European countries [Reference Ashraf17], with birds from the genus Turdus usually suffering the highest mortality rates [Reference Höfle16, Reference Weissenböck18].
In July–October 2013, birds were trapped in the provinces of Huelva, Cádiz and Sevilla (Fig. 1). Birds were captured using mist-nets and subsequently ringed, with sex and age recorded [Reference Svensson19]. Birds were released at the capture site after sampling without injury. A blood sample (volume <1% of body mass) was obtained from the jugular vein of each bird using sterile syringes. Blood samples were maintained at 4 °C for 24 h prior to centrifugation for 10 minutes at 1700 g to separate serum and cellular fractions. Serum samples were frozen at −80 °C until the subsequent virus neutralization test (VNT) was performed. Experimental procedures were approved by the CSIC Ethics Committee on 9 March 2012.

Fig. 1. Place of origin of the avian serum samples analysed in this study (◦) and those with at least one positive sample by ELISA (•). Place of origin of birds with each WNV neutralizing antibody (◆) and flavivirus neutralizing antibody (◇) are shown. The locations with positive cases of WNV infections in horses during 2013 are indicated by ▲.
Initial screening for the detection of antibodies against WNV and other related flaviviruses was performed using the epitope blocking ELISA kit Ingezim West Nile Compac (Ingenasa Spain), which, according to the manufacturer's instructions, requires 10 µl bird serum to measure antibodies [Reference Sotelo20]. Samples giving ELISA positive or doubtful results were subsequently analysed by VNT. For this test we used the micro-assay format (96-well plates) described in the OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals [21] and elsewhere [Reference Figuerola13] with the following modifications: (1) we used Vero instead Vero E6 cells, and (2) the incubation of sample dilutions with viral antigens was performed in the presence of 0·1% bovine serum albumin. The VNTs were performed in the BSL-3 laboratory at CISA in accordance with all current biosafety guidelines. Neutralizing antibody titres were determined in parallel for each serum sample against WNV (strain Eg-101) and USUV (strain SAAR1776) by using serial (twofold) dilutions (1:10–1:1280) of each serum sample in a VNT. Specific responses to viruses were based on the comparison of VNT titres obtained in parallel against the two flaviviruses: the neutralizing immune response observed was considered specific when VNT titres for a given virus were >fourfold higher than the titre obtained for the other virus [Reference Figuerola13].
In all, blood samples from 149 wild birds belonging to 32 different species were analysed in this study (Table 1). With the ELISA kit, positive and doubtful reactions were observed in six and seven individuals, respectively. Only one female juvenile (born in the same calendar year) Sardinian warbler (Sylvia melanocephala) had specific WNV-neutralizing antibodies, with a titre of 1:80. Serum from an adult male blackbird (Turdus merula) neutralized WNV at a titre of 1:40 and USUV at 1:80. These two birds were captured at the beginning of September in the province of Huelva, the former at an equestrian centre and the latter in wetland area.
Table 1. Bird species sampled and analysed for WNV antibodies using ELISA. Positive and doubtful samples using ELISA were subsequently tested using VNT
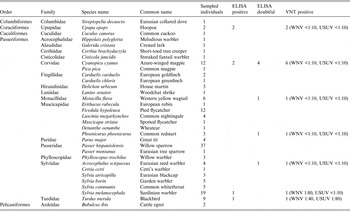
WNV, West Nile virus; VNT, virus neutralization test; USUV, Usutu virus.
We found WNV antibodies in the resident species S. melanocephala. This result supports the idea of local transmission of WNV in southern Spain in 2013, thereby providing more information on WNV transmission dynamics in the area. In 2013, there were WNV outbreaks on horse farms in 34 locations in southern Spain, 28 and six in the provinces of Sevilla and Huelva, respectively (Fig. 1). The closest location with a declared WNV case (S. melanocephala) in horses was 27 km from the capture site, a location with many horses. This indicates that the virus was in fact circulating in a larger area than that suggested by the known cases of disease in horses, and highlights the importance of wild bird surveillance when attempting to detect the circulation of WNV in the absence of the disease [Reference Mannelli22].
Unlike in other bird groups such as rallids [Reference Figuerola23], raptors [Reference Höfle11] and crows [Reference Lim24] (see [Reference Pérez-Ramírez, Llorente and Jiménez-Clavero3] and references therein), only a small proportion of songbirds – the most extensively sampled avian group – were found to have WNV-neutralizing antibodies. Although migration is likely to be an important factor affecting the exposure of avian species to WNV, i.e. trans-Saharan migratory species usually show higher values than migrant species travelling short distances or resident species [Reference López12, Reference Jourdain25], we did not detect WNV antibodies in any migratory species. Possible explanations of these results include inter-annual variations in the proportion of seropositive birds, differences between the species sampled in studies or, simply, the fact that in autumn juvenile birds had not yet migrated to Africa; in fact, in total we only sampled 10 adults of trans-Saharan migratory species (20% of the individuals captured).
Finally, our results strongly support the need to use VNTs to confirm WNV in all positive and doubtful samples detected by ELISA kits in order to increase the accuracy of estimates of pathogen seroprevalence in wild birds. We found that only one of the six ELISA-positive samples reacted in the VNT. The other five birds may have had antibodies that were specific to another flavivirus not studied here such as Marisma Mosquito virus (see [Reference Vázquez26]). Obviously, these results suggest the need for a conservative approach, which will reduce the number of positive individuals. The use of VNT will be especially important in areas where related flaviviruses co-circulate in order to prevent overestimates of the presence of WNV antibodies [Reference Beck5].
ACKNOWLEDGEMENTS
This study was funded by project CGL2012-30759 from the Spanish Ministry of Science and Innovation, project P11-RNM-7038 from the Junta de Andalucía and grant FP7-261504 EDENext. M.F. and J.M.P. were funded by a FPU grant and a Juan de la Cierva contract, respectively. We are particularly grateful for the logistical support provided by the Laboratorio de SIG y Teledetección, Estación Biológica de Doñana, CSIC (LAST-EBD). Special thanks are due to Alberto Pastoriza and Manuel Vázquez for their help during the fieldwork, and to Francisco M. Miranda Castro, Olaya García Ruiz and Carmen Barbero Ameller for their support in the laboratory. Four anonymous reviewers provided valuable comments on a previous version of the manuscript and Mike Lockwood revised the English text.
DECLARATION OF INTEREST
None.





