INTRODUCTION
Campylobacter is a Gram-negative microaerophilic bacterium that can cause human infection resulting in acute bacterial enteric disease [Reference Blaser and Fauci1, Reference Heyman2]. Campylobacter jejuni and C. coli are the most common causes of campylobacteriosis in humans [Reference Takkinen and Ammon3, Reference Friedman, Nachamkin and Blaser4], the former being recognized as a leading cause of bacterial foodborne disease in many developed countries [Reference Moore5]. Apart from enteric disease, Campylobacter infection can cause reactive arthritis and Guillain–Barré syndrome [6–Reference Mishu8].
Among sporadic cases of campylobacteriosis, the most common sources of infection are consumption of raw or undercooked meat, mainly poultry products, consumption of untreated water and contact with pets [Reference Heyman2, 6, Reference Altekreuse, Cohen and Swerdlow9]. The consumption of contaminated water and unpasteurized milk has been associated with outbreaks [6, Reference Tauxe, Nachamkin, Tomkins and Blaser10]. Although Campylobacter outbreaks are relatively rare [Reference Friedman, Nachamkin and Blaser4, 11], waterborne outbreaks have occurred worldwide in many developed countries [Reference Jakopanec12–Reference Allos17].
Campylobacter surveillance in Greece is laboratory based; a sample of hospital laboratories from around the country notify Campylobacter cases confirmed by a positive culture on a weekly basis. This laboratory surveillance system is not mandatory.
On 3 June 2009, the local Public Health Directorate of Chania, a town in western Crete, informed the Hellenic Centre for Diseases Control and Prevention (HCDCP) in Athens about an unusual increase in C. jejuni cases in children living in rural areas around the town. The Chania General Hospital (CGH) informed HCDCP on 5 June 2009 that 31/70 stool samples that had been tested since 29 May 2009 were positive for C. jejuni. According to the laboratory-based surveillance system, the annual number of reported Campylobacter-positive stool cultures in the prefecture of Chania had been two in 2006, nine in 2007 and 13 in 2008. Communications with the paediatric and internal medicine wards of CGH revealed that most of the cases were children and that symptoms were mild, including mainly diarrhoea, often bloody, and fever. Most cases were not hospitalized.
As tap water had previously been identified as the vehicle of a Salmonella Typhimurium outbreak in exactly the same area in February–March 2004, tap water was again suspected at an early stage during the outbreak investigation. Moreover, some patients had been complaining about poor tap-water quality prior to onset of symptoms. In the 2004 outbreak children were the most affected group; 30 (83·3%) of the cases were aged <5 years [Reference Economopoulou18]. Two different water-supply systems exist in the area; one for the municipality of the town of Chania and one for the adjacent rural areas.
Soon after notification of the outbreak, a team from the Office for Foodborne Diseases of the HCDCP visited Chania and started an epidemiological investigation in order to assess the extent of the outbreak and identify the mode and vehicle of transmission.
METHODS
Epidemiological investigation
In order to investigate the risk factors implicated in the outbreak, two analytical studies were conducted in parallel: a case-control study and a case-crossover study.
Case-control study
A frequency-matched case-control study was set up with a 1:2 case:control ratio. Cases were defined as individuals aged 0–14 years that had visited the emergency department of CGH between 27 May and 24 June 2009 with gastroenteritis symptoms and who had a C. jejuni-positive stool culture. Controls were selected from individuals who had visited the emergency department of CGH between 1 January and 31 May 2009 with respiratory tract symptoms. Frequency-matching by age was performed by year of age. Children aged <1 year were matched by 6-month intervals. Parents/guardians of cases were questioned about potential exposures in the 10-day period prior to onset of illness. Parents/guardians of controls were asked about potential exposures during the second half of May 2009. Cases who reported travel outside Chania prefecture in the 10-day period prior to disease onset were excluded because of the potentially unique exposures. Controls who reported travel outside Chania prefecture during the second half of May 2009 were also excluded. Because dietary habits change dramatically with age among toddlers, we analysed data for participants aged >1 year separately. Given that 50 cases had been reported at the time of the inception of the study and that we were expecting a-priori response rates of 60% in cases and 50% in controls, all cases and 124 controls were invited to participate in the study.
The study questionnaire included questions regarding demographic information, clinical symptoms during the interval May–June 2009 and exposure to possible risk factors, such as: consumption of various food items; unique exposures such as participation in common meals or events where food was served; fruit consumption; possession of or contact with animals; history of travel outside Chania prefecture; bathing in the sea, a pool, a river or a lake; use of a dishwasher for children's utensils; use of a microwave in the preparation of children's food and milk; use of a tap-water filter. The questionnaire also included questions on a number of proxies for exposure to tap water: consumption of tap and bottled water at home and at school or in kindergarten; consumption of water from a well, a cistern or a stream; boiling water before consumption; use of a feeding bottle and a pacifier, as well as cleaning and sterilizing techniques; milk types consumed; possible dilution of milk with water; use of tap water for washing fruit, washing teeth, making ice cubes, diluting juices, preparing baby formula; frequency of showering. The questionnaires were completed via telephone interviews with the children's parents during July 2009. Epidata version 3.1 (Denmark) was used for data entry and Stata version 11.0 (StataCorp., USA) for data analysis. Odds ratios (ORs) and their respective 95% confidence intervals (CIs) were calculated. Variables statistically significant at α=0·20 and explaining at least 20% of the cases were included in the multivariable model. In the latter model, an association was considered statistically significant when P⩽0·05; all calculated P values were two-tailed. The multivariable analysis was performed by multiple logistic regression using backwards elimination. We also conducted a stratified analysis, using water-supply system as a potential effect modifier.
Case-crossover study
Case-crossover studies are designed as individually matched case-control studies, with patients serving as their own controls. The exposure for various possible risk factors is examined at two different points in time: (a) during the time directly preceding the outcome of interest (C. jejuni infection) and (b) at an earlier time, or in general a random time interval, during which patients' habits are recorded. Exposures at (a) and (b) are compared for significant differences. Case-crossover studies are not expected to provide statistically significant results for exposures that do not change over time [Reference Maclure19], e.g. tap-water supply system, unless these changed between the two time intervals.
The following definitions were used – cases: as in the case-control study; time interval (a): the 10 days preceding symptom onset for each case; time interval (b): spring 2009 until 10 days before symptom onset.
We used the same questionnaire as in the case-control study with the exception that, in the case-crossover study, the same questions were asked twice for cases: once for time interval (a) and once for time interval (b). The analysis was performed via conditional logistic regression and McNemar's χ 2 test. Statistical significance was defined as in the case-control study.
Laboratory investigation
Campylobacter spp. testing is a routine procedure for stool cultures of children aged <5 years at CGH, whereas, for children aged ⩾5 years, it is only performed when the standard cultures for Salmonella and Shigella are negative. Due to the sudden increase of the C. jejuni-positive stool cultures in children, it was suggested that Campylobacter cultures should be performed for all children regardless of age, as well as for every third adult patient visiting the emergency department of CGH with symptoms of gastroenteritis from 3 June 2009 until the end of the outbreak. In addition, all isolates were sent to the Laboratory of Clinical Microbiology, Parasitology, Zoonoses and Geographical Medicine for performance of pulsed-field gel electrophoresis (PFGE) and multi-locus sequence typing (MLST). PFGE was performed in all samples according to CDC protocol [Reference Ribot20]; the enzyme used was SmaI. MLST was performed in three genes for three randomly chosen samples.
Environmental investigation
In the week of 8 June 2009, the Public Health Directorate of Chania prefecture performed bacteriological testing of the water-supply system of the Municipality of Chania, as well as of the systems of adjacent rural municipalities, where the outbreak had occurred. All water samples were taken from randomly selected points in the two water distribution systems and were tested according to ISO 17025. In addition, the history of water sampling and subsequent chlorination actions of the municipalities of the area were reviewed for the weeks preceding the outbreak's onset. All results available from water-quality testing performed by the Municipal Establishment of Water Supply and Drainage of Chania district since 1 January 2009 were reviewed. CGH is supplied by the same water distribution system as the rural areas, where the outbreak occurred, and regularly tests tap-water quality at different points in its distribution system. The results of these independent CGH controls were also collated and reviewed. Extra chlorination was performed by the local municipal authorities. Bottled water and chicken from supermarkets in the outbreak area were sampled for the week of 8 June 2009 and tested for indicator microorganisms and presence of C. jejuni at the Central Laboratory of Public Health, Athens, according to ISO 6222:1999, ISO 6340:1995, ISO 6461-1:1986, ISO 7889-2:2000, ISO 8199:2005, ISO/CD 9308-1, ISO 9308-1:2000, ISO 9308-1:2000/Cor 1:2007 and ISO 17995:2005. Chicken samples were tested for Campylobacter according to ISO 10272-1:2006. PFGE and MLST were run on any Campylobacter-positive chicken sample, as described above.
Ethical approval
The study was approved by the CGH board of directors (decision no. 288/16 June 2009). Informed consent was obtained from the parents or guardians of the patients.
RESULTS
Descriptive results
Sixty cases had a positive C. jejuni stool culture confirmed at CGH between 1 May and 24 June 2009. Fifty-five of these cases (91·7%) resided in the rural areas adjacent to the town of Chania and the remainder resided within the town's boundaries. For 54 of the cases, the exact date of sample acquisition was known (Fig. 1). Of 59 cases of known sex, 27 (45·8%) were female. Fifty-nine cases also had their age registered; the median age was 2·1 years (36 days to 73 years). In total, 7/59 (11·9%) cases were aged >14 years. Of all these cases in the outbreak area, 50 were eligible for participation in the analytical studies.
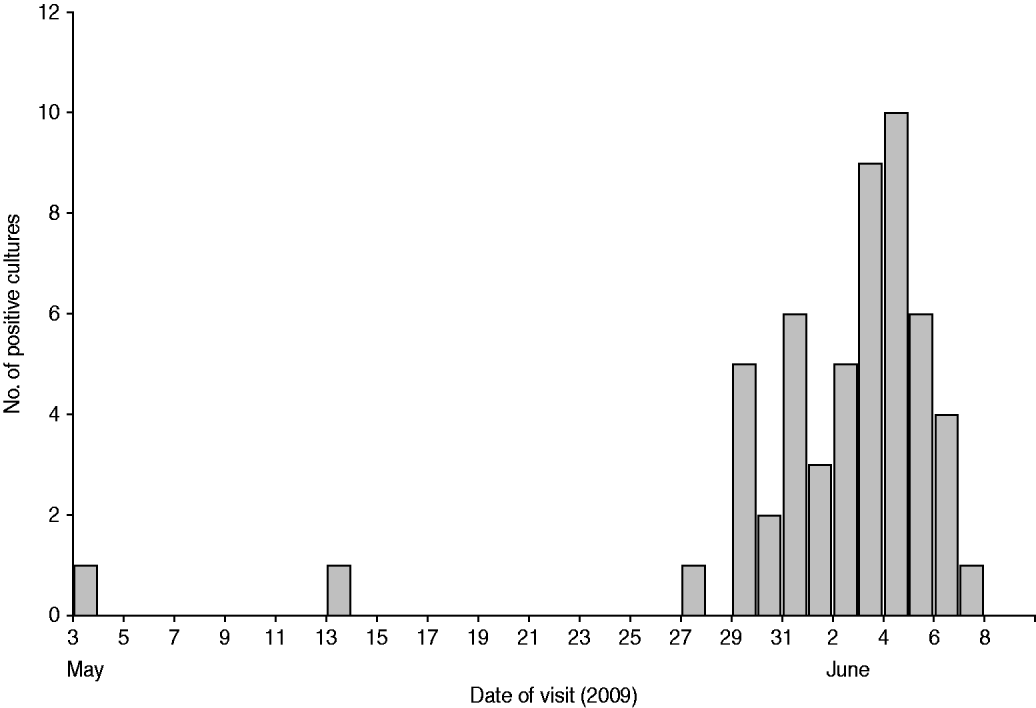
Fig. 1. Positive Campylobacter jejuni stool cultures by date of visit, Chania General Hospital, May–June 2009 (n=54).
Thirty-seven cases and 79 controls responded (response rates 74·0% and 63·7%, respectively). The median age of both cases and controls was 2 years (1 month to 10 years, and 2 months to 11 years, respectively). Sex distribution did not differ between cases and controls; 17 (45·9%) of the cases and 38 (48·1%) of the controls were female. Thirty-five (94·6%) of the participating cases resided in the rural areas. Of all 116 participants, 31 (26·7%) resided in areas where the water was supplied by the municipal water company of Chania. No cases or controls reported travel. Sex and age distribution did not differ between respondents and non-respondents. The most commonly reported symptoms in cases were diarrhoea (100%), fever (58·3%), bloody stool (58·3%), vomiting (27·8%) and abdominal pain (25·0%). No other symptoms were reported. Vomiting was present only in children aged >1 year.
Analytical studies
Case-control study
Univariate analysis
Several exposures were associated with C. jejuni infection. Consumption of tap water from the rural areas' supply system was a risk factor for C. jejuni infection (OR 4·35, 95% CI 1·30–16·7). Consumption of bottled water (OR 0·16, 95% CI 0·04–0·51), the existence of a water filter (OR 0·00, 95% CI 0·00–0·47) and using a dishwasher for children's utensils (OR 0·11, 95% CI 0·03–0·48) were protective factors. Table 1 presents statistically significant associations at the 0·20 level. The results of the separate analysis for participants aged >1 year were very similar to those for children aged <1 year; the only notable difference was that for children aged ⩾1 year, consuming baby formula that had been prepared with tap water had an OR of 0·36 (95% CI 0·11–1·09). This finding was not statistically significant in infants aged <1 year.
Table 1. Risk and protective factors for C. jejuni infection in the greater Chania area, May–June 2009, univariate case-control study and case-crossover study results
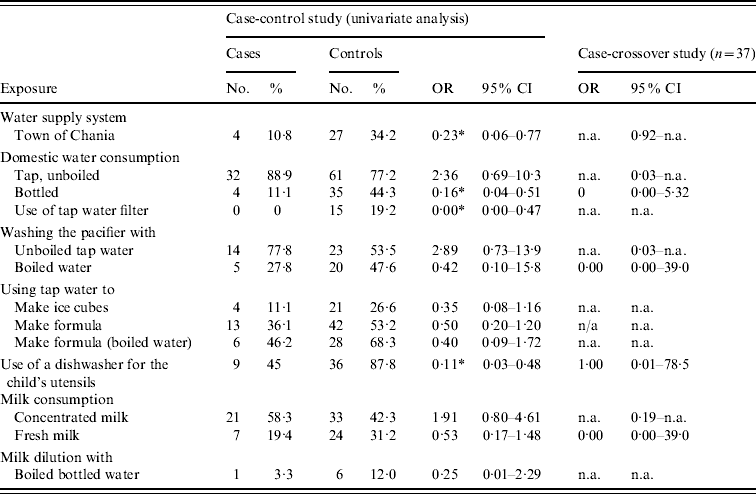
OR, Odds ratio; CI, confidence interval; n.a., not available.
* OR differing statistically significantly from 1 (P⩽0·05).
Stratified analysis
We also stratified the analysis by tap-water supplier, a potential effect modifier. The OR for consumption of unboiled tap water at home could be computed for rural areas only (OR 3·11, 95% CI 0·86–14·0). Similarly, consumption of bottled water and the use of a tap-water filter were protective factors only in rural areas (OR 0·15, 95% CI 0·04–0·54 and OR 0·00, 95% CI 0·00–0·53). The use of a dishwasher for children's utensils was protective against infection in both rural and urban areas. We grouped all milk types requiring dilution with water (concentrated milk, powder milk) into a new variable. Consumption of such milk types was found to be a risk factor only in rural areas (OR 3·48, 95% CI 1·06–13·3). Table 2 presents statistically significant associations at the 0·20 level in the stratified analysis. These findings suggest that the effect of tap-water consumption differed between rural and urban areas.
Table 2. Risk and protective factors for C. jejuni infection in the greater Chania area, May–June 2009, stratified analysis results
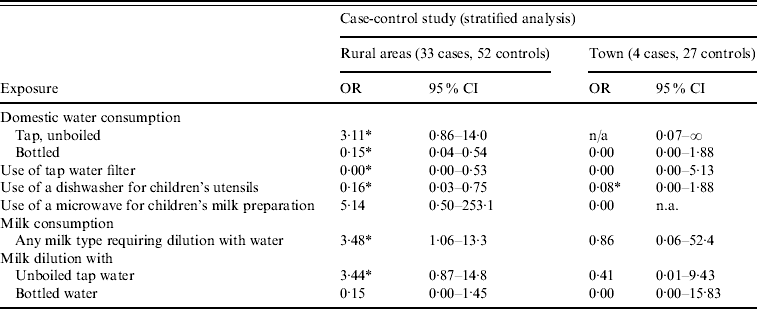
OR, Odds ratio; CI, confidence interval; n.a., not available.
* OR differing statistically significantly from 1 (P⩽0·05).
Multivariable analysis
In the multivariable analysis, exposure to the water-supply system of rural areas and drinking tap water were statistically significantly associated with C. jejuni infection; adjusted ORs and 95% CI were 5·88 (1·75–20·0) and 4·39 (1·30–14·8), respectively.
Case-crossover study
No risk factors were found to be statistically significant in the case-crossover study (Table 1).
Laboratory results
Forty-nine C. jejuni-positive stool samples from patients were cultured in Campylobacter agar. All Campylobacter isolates were sensitive to erythromycin. PFGE results showed that 48/49 stool samples were identical to each other. One was different, but there was some doubt regarding the purity of the culture or bacteria identity (Fig. 2). MLST results were identical.

Fig. 2. Some of the identical Campylobacter jejuni samples (lanes 34, 37–40, 42–47, 49). The one different sample is seen in lane 48 (see text). The chicken sample is shown in the column labelled CH and is distinguishable, like lane 48. The marker can be seen on the far left (all patterns produced using the SmaI enzyme).
Environmental results
Potable water is automatically chlorinated at municipal level before distribution to households in all municipalities around Chania. There had been no power outages during the time preceding the outbreak that could have led to temporary poor water chlorination. In non-outbreak settings, municipalities are not obliged to run full water controls more than once per year. Some of the tests carried out in one of the outbreak municipalities showed total absence of residual chlorine in tap water at a site within the outbreak area. However, results were consistently >0·2 mg/l within the municipality of Chania. During the time prior to outbreak onset, the hospital had repeatedly found very low residual chlorine levels in its tap water; in one of the sites tested in the hospital, residual chorine levels were as low as 0·04 mg/l. Tap and bottled water tests were negative for Campylobacter and indicator microorganisms.
Three chicken samples were taken from three supermarkets and one was found to be positive for C. jejuni. PFGE results from the positive chicken culture were different compared to those from the stool samples (Fig. 2). The MLST results were also different.
DISCUSSION
An outbreak of C. jejuni took place around the town of Chania in late May to early June 2009. This is the same area where a S. Typhimurium outbreak had occurred in February–March 2004. Again, it was only the rural areas around the town of Chania that had been affected [Reference Economopoulou18]. The analytical study in 2004 had provided epidemiological evidence that tap water had been the vehicle of the outbreak. The 2004 outbreak followed an unusually heavy snowstorm on the island; it had been speculated that frozen water pipes might have caused leakages, thus contributing to the outbreak.
Five years later, in late spring 2009, tap-water consumption in the same areas was suspected in the acquisition of C. jejuni infection. In the case-control study, we found that tap-water consumption was a risk factor for acquiring C. jejuni infection. This finding was consistent in many variables we used as tap-water consumption proxies univariately (Table 1). The consumption of baby formula was found to be a protective factor in children aged ⩾1 year, although the formula was prepared with boiled water.
More interestingly, stratified analysis showed that the association between C. jejuni infection and tap-water consumption, as well as their proxies, differed by water-supply system. Consumption of bottled water and the use of a tap-water filter or a dishwasher for children's utensils at home were protective only in rural areas; moreover, only in rural areas was consumption of any type of milk requiring dilution with water found to be a risk factor. These results clearly show effect modification and are compatible with the acceptable water-quality test results within the town of Chania and the very few cases originating from the town compared to its surrounding rural areas.
Even though most cases originated from rural areas, we also included cases from the town in our analysis. By doing so, we were able not only to describe all C. jejuni cases, but also to identify the different exposures to which cases from the two different areas were exposed. Having chosen not to include the cases from the town of Chania in our analysis, we would not have been able to test if place of residence, or indeed water-supply system, were effect modifiers in this outbreak.
Although there is strong epidemiological evidence that tap water was the vehicle of this outbreak, no Campylobacter was found in any of the environmental samples tested. Unfortunately, the alleged hyperchlorination of water at the municipal level in rural areas prior to sampling may have contributed to the failure to find an environmental link in this outbreak. Furthermore, it did not become clear from the outbreak investigation, and the interviews with the local officials, how the bacteria could have been introduced into the water distribution system. The epidemic curve (Fig. 1) is suggestive of a point-source outbreak; the most probable time of exposure to contaminated water was 25–28 May 2009, a period prior to or during which no extreme weather had been reported in the area. It is possible that the low documented water chlorination levels contributed to the survival of bacteria in the water distribution system for some time; by Greek law, tap-water chlorination should result in residual chlorine levels of at least 0·2 mg/l in the end product. Furthermore, the fact that molecular techniques revealed identical PFGE patterns and DNA sequences in human isolates implies that tap-water contamination took place from a single source. Full mapping of the regional water distribution system and more exhaustive testing have been recommended to the local authorities and this should help to identify any points where bacteria are introduced into the system.
From the available data, infants and toddlers appeared to have been more affected than other age groups. The fact that Campylobacter cultures were performed only in the younger age groups presenting with diarrhoea at CGH at the early stages of the outbreak may have biased the age distribution of cases towards the lower ages. However, other researchers have found that infants become sick more easily than adults when infected with Campylobacter [Reference Taylor21, Reference Rao22]. Moreover, infants tend to change their diet as they grow, thus becoming exposed to different kinds of food items, including non-maternal milk and commercial milk types that require dilution with water prior to consumption. Despite the possible bias in the cases' age distribution, frequency-matching for age in the controls' selection has reduced the risk of selection bias.
The use of a dishwasher for children's utensils, was found to be a protective factor for C. jejuni infection univariately and in the stratified analysis. The U.S. Food and Drug Administration Food Code specifies that the temperature of the washing zone in manual washers should be ⩾43°C [23]; however, this condition is not always met because it can feel uncomfortable, and a documented reluctance to wash utensils at such high temperatures [Reference Pfund24]. More researchers have commented on manual dishwashing being prone to fluctuations in its efficiency to remove microorganisms [Reference Mattick25]. Using dishwashers may thus have a protective role in the spread of bacteria within the kitchen.
Apart from the case-control study, a case-crossover study was also performed using the same questions on possible exposures; we found no statistically significant associations between the investigated exposures and C. jejuni infection. Case-crossover studies are expected to provide no epidemiological evidence for exposures that do not change over time. Certainly, this study design would not be the analytical epidemiological method of choice for this outbreak investigation. However, the results of the case-crossover study strengthen the hypothesis that no food items or unique exposures, such as dietary risk factors linked to early summer, were found to be associated with C. jejuni infection. The results of this study should be interpreted along with those of the case-control study.
It could be argued that a possible limitation of our study was the possible presence of recall bias, as interviews were held in July 2009, a maximum of about 1½; months after symptom onset. However, parents tended to have good recall regarding the source of water used for consumption in their household; it may be unlikely that parents who use tap water for their children's utensils and food preparation changed to bottled water for no apparent reason. However, a total absence of recall bias cannot be claimed.
Municipalities are not obliged to control water quality more than once per year. Even though some monitor water quality more often, this may not be enough to timely detect water-quality deficiencies. All results of the current outbreak investigation have been communicated to all competent bodies and awareness has been raised of the need for closer cooperation between the local public health directorate, municipalities and local domestic water suppliers regarding the usefulness of more regular water testing and full mapping of the water distribution system. Stricter laws for water testing nationally would also help towards prevention of future waterborne outbreaks.
ACKNOWLEDGEMENTS
We thank Effie Kalogeraki, Stavroula Marakaki and Ioanna Simantiraki from the Local Public Health Directorate of Chania for their assistance throughout the outbreak investigation; Georgia Pavlidaki and Kaiti Tsafaraki from CGH for their efforts in facilitating communications and being a contact point between HCDCP and clinicians and laboratory experts in the hospital; and Georgios Theocharopoulos, Georgios Dougas, Eleni Lyllakou and Costas Danis for participating in the interviews of cases and controls.
DECLARATION OF INTEREST
None.






