INTRODUCTION
Prevalence of hepatitis B virus (HBV), hepatitis C virus (HCV) and human immunodeficiency virus (HIV) in the general population of Croatia is about 8–11%, 0·8–1·3% and 0·002%, respectively [Reference Lesnikar1, Reference Gjenero-Margan and Kolarić2] and it is much higher in high-risk populations such as intravenous drug users (IDUs) (35%, 56%, 0·8%, respectively) and men that have sex with men (MSM) (29·6%, 7·3%, 4·6%, respectively) [Reference Gjenero-Margan and Kolarić2, Reference Kribl, Miškulin and Bojanić3]. The prevalence of these viruses in Croatian prisons is unknown but the structure of prisoners in correctional facilities indicates that it must be high. Our preliminary results indicate that IDUs represent the greatest risk population in prisoners. Moreover, it is well known that a high proportion of prisoners in many countries inject drugs which increases the possibility of virus transmission inside the prison setting [Reference Malliori4–Reference Dolan, Hall, Wodak, Ward, Mattick and Hall6].
We present the results of a national study examining the structure of prisoners in Croatia, the prevalence of antibodies to HBV, HCV and HIV among them, the prevalence of HBV/HCV co-infection, the percentage of acute HBV and HCV infections occurring within prisons and the percentage of vaccinated prisoners.
METHODS
Study population
We conducted our survey on prisoners and persons in custody. According to the Ministry of Justice – Department of Prison System, in 2007 there were 10 378 individuals [10 186 adults (9424 men, 762 women), and 192 juveniles (175 men, 17 women)] in 20 prisons. Correctional staff (n=2385; 1743 men, 642 women) were also included in this project because they are potential risk groups for occupationally acquired infections with bloodborne pathogens. According to the regulation of the Ministry of Justice, Department of Prison System, prisoners aged ⩽18 years are treated as juveniles.
The survey was carried out between December 2005 and December 2007 and comprised two parts: (i) a questionnaire and (ii) the collection of blood samples. Researchers met groups of 10–25 prisoners. Staff and prisoners were briefed in advance. The survey was explained, and prisoners were advised that the survey was voluntary, anonymous, and confidential. No negative sanctions were imposed on non-respondents. Once completed, the questionnaire and specimens were placed in a sealed envelope and numbers were assigned to both, linking them.
The questionnaire consisted of questions relating to demography, prison sentence, risk behaviours, self-reported hepatitis and HIV testing and hepatitis B vaccination. It was self-administered and took about 5 min to complete.
Serological markers for HBV, HCV, and HIV infection
Blood samples were collected with an appropriate device and transported to the laboratory in batches on the same day by courier. Processing started the next day and the samples (separated serum from coagulum), stored at −20°C, were tested in groups of 96 every 1–2 weeks.
Serological markers used for HBV infection were: HBsAg, anti-HBc, anti-HBs and IgM anti-HBc. For HCV infection we used anti-HCV and HCVAg-Ab. All reactive HBsAg was confirmed by HBsAg neutralization test and anti-HCV positivity with recombinant immunoblot (RIBA). Acute HBV infection was based on IgM anti-HBc positivity. For many years IgM anti-HBc has been considered a specific marker of acute HBV infection. However, in a small percentage of samples, when using more sensitive methods, a low level of this antibody could be found during chronic infection (chronic inactive carrier, asymptomatic patients suffering from chronic hepatitis B) [Reference Rodella7]. In our study all IgM anti-HBc positives had a level of positivity >200 OD. Therefore it could be seen that this was a clear sign of acute HBV. Acute HCV infection was based on anti-HCV seroconversion and/or development of more lines on Western blot analysis in paired sera within an interval of 1–3 months. All of the tested prison population were clinically without symptoms. Every individual with positivity for any of the HBV markers (HBsAg, anti-HBc, anti-HBs) or anti-HCV was tested by AST and ALT and those with positive HBV markers were additionally tested with IgM anti-HBc. Individuals with HBsAg negativity and anti-HBc/anti-HBs positivity, all normal by AST and ALT, and IgM anti-HBc negativity were treated as past HBV infections. Those who were IgM anti-HBc positive (with higher or normal AST and ALT levels) were treated as acute HBV infections (HBsAg positive or HBsAg negative acute HBV). Individuals that seroconverted from anti-HCV negativity to anti-HCV positivity and those with only one or a few lines on Western blot, who developed more lines (i.e. those with two serum samples over 1–3 months) were treated as acute HCV infections.
RESULTS
The survey was conducted in 20 prisons throughout Croatia. We analysed blood samples of 3348 adults (3160 men, 188 women), 144 juveniles (130 men, 14 women) and 259 members of correctional staff (201 men, 58 women) – which represents 32·9% of the total Croatian adult prisoner population, 72·9% of the total juvenile prisoner population, and 10·9% of total prison staff. A total of 654 prisoners gave blood twice. The first blood sample was taken 3 months after entry to prison and the time interval for the second sample was between 3 and 6 months.
The median age (range) of respondents was 32 (21–70) years for adults and 18 (16–20) years for juveniles.
Structure of prison population
Adults
The most prevalent risk group for adults was the IDU group (24·3%) while a relatively high percentage were in the alcoholic (11·9%) and highly promiscuous (8·2%) groups. Individuals with personal abnormalities and with psychiatric diseases comprised 5·8% and 2·9%, respectively. According to the questionnaire only 0·2% declared themselves to be in the MSM group and those who were at no risk comprised 41·9% (Table 1).
Table 1. Structure of the adult prison population according to risk groups and gender

IDU, Intravenous drug user; MSM, men who have sex with men; PTSD, post-traumatic stress disorder.
Adults males comprised the highest percentage in the IDU group (24·7% vs. 19·2%) and females predominated in the highly promiscuous group (12·7% vs. 7·9%) (Table 1).
Juveniles
Contrary to adults the most prevalent high-risk group in juveniles was the highly promiscuous group (22·9%). The alcoholic (13·2%) and IDU (12·5%) groups had a similar percentage and there was a relatively high number of individuals with personal abnormalities (8·3%). The percentage of juveniles at no risk was similar to that of adults (38·2%).
Regarding juveniles males predominated in the highly promiscuous group (23·8% vs. 14·3%) and females predominated in the IDU (35·7% vs. 10·0%) and alcoholic (21·4% vs. 12·3%) groups (Table 2).
Table 2. Structure of juvenile prison population according to risk groups and gender

IDU, Intravenous drug user; MSM, men who have sex with men.
Prevalence of HBV markers
Adults
In the prison population the total HBV infection in men and women is similar (16·5% vs. 16·0%, respectively). The highest percentage is found in the IDU risk group (27·3% men vs. 25·0% women). For males high positivity was found in the following groups: psychiatric diseases (19·0%), highly promiscuous (19·0%), non-IDUs (16·3), alcoholics (13·7%) and personal abnormalities (13·8%). For women the highest positivity was found in the IDU (25·0%) and alcoholic (27·3%) groups, followed by the highly promiscuous (21·7%) and psychiatric diseases (16·7%) groups. The most prevalent combination of HBV markers was HBsAg negative, anti-HBc positive, anti-HBs positive (7·4%; both men and women). A high percentage was found with isolated anti-HBc positivity (anti-HBc alone) (5·8% men, 5·9% women). A relatively high percentage of HBsAg chronic carriers was found in the male population (1·3%) especially in individuals with psychiatric diseases (3·6%) and alcoholics (2·1%) and non-IDUs (2·0%) (Table 3). Isolated anti-HBc and co-occurring IgM anti-HBc negativity were treated as a past infection.
Table 3. Prevalence of HBV, HCV and HIV markers in all Croatian prisoners according to risk groups and gender
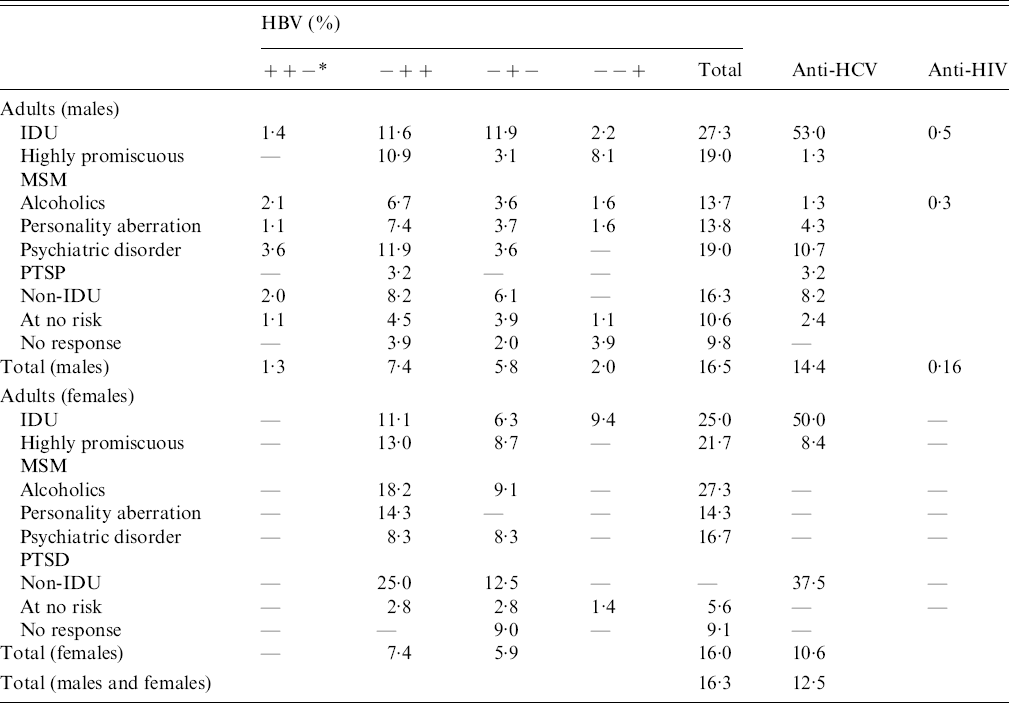
IDU, Intravenous drug user; MSM, men who have sex with men; PTSD, post-traumatic stress disorder.
* Three symbols mean respectively: HBsAg, anti-HBc, anti-HBs.
Juveniles
Total HBV infection in male juveniles was 18·9% with the highest percentages being found in males in the highly promiscuous (44·8%), alcoholic (25·0%), and IDU (23·1%) groups. HBsAg chronic carriers were relatively high (1.6%), with the highest percentage in the alcoholic group (6·3%) (Table 4). In female juveniles HBV infection was registered in only 20% of the IDU group.
Table 4. Prevalence of HBV, HCV and HIV markers in juvenile Croatian prisoners according to risk groups and gender

IDU, Intravenous drug user; MSM, men who have sex with men; PTSD, post-traumatic stress disorder.
* Three symbols mean respectively: HBsAg, anti-HBc, anti-HBs.
Prevalence of HCV markers
Adults
Total anti-HCV positivity was found in 14·4% of men and 10·6% of women prisoners. The highest percentage of HCV infection was registered in the IDU group (53·0% men, 50·0% women). For males a very high percentage of HCV infection was found in the following groups: psychiatric diseases (10·7%), non-IDUs (8·2%) and individuals with a personal aberration (4·3%). In the female population a high prevalence of anti-HCV was found in the highly promiscuous group (8·4%) (Table 3).
Juveniles
In juveniles HCV infection was found only in the male population (4·3%) with 23·1% in the IDU group (Table 4). No HCV infection was registered for females.
Prevalence of HIV markers
HIV testing revealed only 0·15% anti-HIV positives in the adult male population (5/3348), with 0·5% in the IDU group and 0·3% in the alcoholic group.
Correctional staff
Testing of HBV markers in unvaccinated staff showed 11·8% positivity (13·4% men, 10·3% women). All samples were negative for HCV and HIV (Table 5).
Table 5. Prevalence of HBV, HCV and HIV markers in correctional staff according to gender

* Three symbols mean respectively: HBsAg, anti-HBc, anti-HBs.
HBV/HCV co-infection
Adults
HBV and HCV co-infections were found in 6·1% of all adult prisoners, with more in the male (6·2%) than the female (4·3%) population. Of the HBV-positive individuals the percentage of those with HCV co-infection was 34·9–35·3% in men and 26·7% in women (Table 6).
Table 6. Simultaneous HBV and HCV infection in adult Croatian prisoners according to age and gender

* Three symbols mean respectively: HBsAg, anti-HBc, anti-HBs.
Juveniles
HBV/HCV co-infection was not registered in juveniles.
Spread of HBV, HCV and HIV infection inside prison: acute infection
HBV infection
IgM anti-HBc positivity was found in eight out of 3348 prisoners and seroconversion (anti-HBc negative on first testing and positive on second blood sample) in two persons in a group of 654 individuals with two blood samples taken.
HCV infection
Seroconversion (anti-HCV negative on first testing and positive on second blood sample) was registered in five persons in a group of 654 individuals with two blood samples taken. An increasing number of antibodies to HCV antigens was found in three individuals already anti-HCV positive in the same group of 654 individuals (Table 7).
Table 7. Spread of HBV, HCV and HIV infection inside prison – acute infection during 1-year period

HIV infection
Of 654 individuals with two blood samples taken there was no seroconversion from anti-HIV negative to anti-HIV positive.
Hepatitis B vaccination in prisoners and correctional staff
Adults
The vaccinated population in adult prisoners is extremely low at 4·3%. Of the 1247 respondents who answered the question 38 (3·1%) stated they had completed a three-dose course of HBV vaccination, 15 (1·2%) received one or two doses and 1194 (95·7%) had not received any vaccine. We found a response to HBV vaccination in only 22·6% of individuals (20·0% men, 66·6% women).
Juveniles
The vaccinated population in juvenile prisoners represents 29·9% (29·3% men, 50·0% women). A total of 77 individuals answered the question and 20 (26·0%) had completed a three-dose course, three (3·9%) had received one or two doses, and 54 (70·1%) had not received HBV vaccine. Anti-HBs was registered in 54·2% of individuals (52·2% men, 100·0% women).
Staff
A high proportion of staff (69·5%) were vaccinated (71·4% men, 62·8% women) and a high response to HBV vaccine (71·0%) was registered (73·1% men, 62·5% women). The majority (159, 37·0%) received a three-dose course of HBV vaccine, only 1·3% (n=3) received one or two doses and 60 (25·7%) had not received any vaccine (Table 8).
Table 8. HBV vaccination in prisoners and correctional staff

DISCUSSION
Structure of the prison population
In the heterogeneous structure of the prison population in Croatia the most numerous (with the exception of the no-risk group) are IDUs – almost one quarter (24·3%) with higher prevalence in males than females (24·7% vs. 19·2%). This percentage is similar to that found in Ireland [Reference Long8] but is much lower than in the majority of European countries with a prevalence of 30–68% [Reference Malliori4, Reference Tefanova, Tallo and Kutsar9–Reference Cristensen14]. It is followed by the alcoholic group for men (12·2%) and the highly promiscuous group for women (12·7%). In the risk groups in the juvenile population the IDU group is in third place (12·5%) behind the highly promiscuous (22·9%) and alcoholic (13·2%) groups. This national survey, which covers all prisons throughout Croatia (n=20), shows that around one quarter (25·7%) of Croatian prisoners are infected with HBV, HCV or both viruses.
HBV infection
In the general population of Croatia the prevalence of HBV infection is about 11·0% [Reference Lesnikar1, Reference Gjenero-Margan and Kolarić2]. The percentage of HBV infection in the adult prison population in Croatia (16·3%) is in accord with the majority of reports where the prevalence of serological markers for current or past HBV infection in prison inmates ranges from 13% to 47%, and varies by region [Reference Kribl, Miškulin and Bojanić3, Reference Dolan, Hall, Wodak, Ward, Mattick and Hall6, Reference Long8–Reference Butler13]. It is comparable to that in Denmark (10–20%) [Reference Cristensen14] and England (10%) [Reference Weild15], is higher than that in Ireland (8·7%) [Reference Long8], on the lower limit of some reports in USA (13–47%) [16] and much lower than in France (32%) [Reference Rotily17], Greece (49·0%) [Reference Giotakos18] and Italy (52·7) [Reference Babudieri10]. This high percentage of HBV infection is primarily due to high HBV infection in IDUs (26·2%) as is the case in other countries where it ranges from 18% to 80% [Reference Malliori4, Reference Long8, Reference Pallas11, Reference Cristensen14–Reference Giotakos18]. Prevalence of HBV infection is also high in males in the highly promiscuous (19·0%) and psychiatric diseases (19·0%) groups. In female prisoners, after the IDU group, HBV infection is found to be higher than in the general population in the alcoholic (27·3%) and highly promiscuous (21·7%) groups and significantly so in the psychiatric diseases group (16·7%). The percentage of HBV infection, greater than in general population in the last three groups, could be explained by different factors, the most likely being prostitution, unprotected sexual intercourse and higher injecting drug abuse, especially in the psychiatric group [Reference Turpeinen19].
Juveniles
The majority of juvenile offenders have behaviours that place them at risk for HBV infection (e.g. injection-drug use or unprotected sex with multiple partners). In the juvenile population the percentage of HBV infection is higher than in adults (18·9%) primarily because of great positivity in the highly promiscuous (44·8%) and alcoholic (25·0%) groups in males, and is higher than in England (4%) [Reference Weild15] and USA (0–6%) [16]. All HBV positivity in juvenile females is concentrated in the IDU group (20·0%).
Combination of HBV markers
HBsAg positivity (together with anti-HBc positivity) without clinical symptoms and AST/ALT elevation (chronic HBsAg carriers) reflects high infectivity and potential of HBV transmission (depending, naturally, on HBeAg/anti-HBe status). Prevalence of HBsAg positivity in Croatia (1·3%) is similar to that in England (1%) [Reference Weild15] and France (1·3%) [Reference Rotily17]. A much higher percentage was found in prisoners in Greece, Italy, Australia and Belgium (3·1%, 3·2%, 4·4%, 6·5%) [Reference Malliori4, Reference Babudieri10, Reference Butler20, Reference Todts21]. HBsAg positivity was not found in female prisoners tested in Croatia.
The combination of three HBV markers (HBsAg, anti-HBc, anti-HBs) shows a high percentage (5·8%) of isolated anti-HBc positivity (anti-HBc alone), which is similar to the report from France (6·4%) [Reference Rotily17]. Isolated anti-HBs positivity is based on the exclusion of HBV vaccinated individuals (2·0%) and is lower than that in the Irish report where 8·7% of prisoners had evidence of non-vaccine-induced antibodies to hepatitis B [Reference Allwright22].
Men vs. women
Our data show slightly lower incidence of HBV infection in females (16·0%) than in males (16·5%). However, in the majority of reports prevalence of HBV markers in adult prisoners is higher in females than in males (USA: females 37–47% vs. males 13–32%) [Reference Weinbaum, Sabin and Santibanez23], England (12% vs. 8%) [Reference Weild15], Germany (13% vs. 7%) [Reference Keppler, Nolte and Stöver24].
The greater proportion of women might be explained by IDU and prostitution before imprisonment and as a result of more frequent contact with healthcare services for routine gynaecological examinations which gives female offenders greater opportunities for disease testing compared to their male counterparts.
HCV infection
Adults
The total percentage of HCV infection in adult prisoners (12·5%) in comparison with the general population (1·2%) is very high (14·4% men, 10·6% women) as is the case with HBV infection primarily due to the IDU group (53·0% men, 50·0% women). There is a surprisingly high percentage of anti-HCV positivity in the psychiatric diseases (10·7%) and non-IDU (8·2%) groups for men, and non-IDU group (37·5%) for women. As is the case with HBV infection, the higher percentage of positivity in the psychiatric group might be explained by higher injection drug abuse, prostitution and unprotected sexual intercourse, while in the non-IDU group the explanation might be due to incorrect answers of examinees, and in the female population the small absolute numbers tested. The HCV positivity of 12·5% in Croatian prisoners is on the lower limit of that in the USA (16–41%) [16, Reference Allwright22] and much lower than in Ireland (37%) [Reference Long8] and Scotland (20·3%) [Reference Gore25]. The percentage HCV positive in the IDU group in Croatian prisons (51·5%) is lower than in the majority of other reports ranging from 56% to 74·8% [Reference Long8, Reference Tefanova, Tallo and Kutsar9, Reference Butler13–Reference Butler20, Reference Allwright22, Reference Weinbaum, Sabin and Santibanez23].
Juveniles
There is only very limited data available on the prevalence and course of HCV in young offenders. In the juvenile population overall anti-HCV positivity is much lower than in adults (4·3% males) and it is concentrated in the IDU group (23·1%). In a report from Germany 8·6% of juveniles were positive for anti-HCV [Reference Meyer26]. This percentage is similar to that found in the USA (2–3·5%) [16]. Regardless of reported risk behaviours, the prevalence is higher in females than in males (3–7% vs. 2–3%) [16].
Men vs. women
As is the case with HBV infection we did not find a greater difference in prevalence of HCV infection between men and women, contrary to the majority of reports from other countries where the female population had a greater percentage of HCV positivity. Women are reported to have greater variability than men in the prevalence rate. Studies done in Canada, USA and Australia have shown that the prevalence of HCV in females ranges from 25·3% to 67·0%, comparable with 4·0–39·4% in males [16, 27, Reference Butler28]. Butler et al. reported that the higher rate in females was the result of a higher concentration of female prisoners for drug-related offences [Reference Butler13].
Staff
Correctional staff are among groups at potential risk for occupationally acquired infections with bloodborne pathogens. As expected, prevalence of HBV markers in staff (11·8%) is similar to that in general population – probably due to the high percentage of HBV vaccination, and is in accord with a report from the USA (12·6%) [Reference Butler28]. There are few data about HCV infection among staff. We have not registered HCV infection in correctional staff and our findings of 0% are similar to unpublished studies in the USA (2%), therefore it is not higher than in the general population. These findings are similar to those of studies in other occupational groups, including hospital-based healthcare workers, surgeons, and public safety workers [Reference Averhoff29, 30].
Co-infection
In incarcerated persons, shared risk factors (e.g. injecting-drug use) can result in populations co-infected with HBV, HCV or HIV. Of 582 HBV-positive individuals (with various constellation of HBV markers) 34·9% were co-infected with HCV and on the level of total number of the tested prison population (n=3348) this percentage is 6·1% (6·2% men. 4·3% women). This relatively high percentage of HBV/HCV co-infection is very important because this dual infection progresses more frequently and faster to chronic sequelae – cirrhosis and hepatocellular carcinoma and co-infections can make treatment of chronic viral hepatitis, AIDS and tuberculosis more difficult because of the need to use multiple drugs, which increases the chance of hepatotoxicity and other adverse events [31, 32]. HBV/HCV co-infection in Croatian prisoners (34·9%) is much lower than that found in Austria (59%) [Reference Michalak, Pham and Mulrooney-Cousins33] and Estonia (65%) [Reference Tefanova, Tallo and Kutsar9].
Transmission of acute HBV and HCV infection
Adults
The majority of HBV and HCV infections in incarcerated persons are acquired in the community. However, correctional facilities are not isolated communities. Prisons are ideal places for transmitting infectious disease because of the environmental conditions, the high concentration of HBV-, HCV- and HIV-infected individuals and the occurrence of risk behaviour (injection drug use, consensual sex, rape and tattooing with contaminated equipment) [Reference Cristensen14, Reference Weild15, Reference Todts21, Reference Gore25, Reference Arrada, Zak Dit Zbar and Vasseur34, Reference Hammett35]. Transmission can also occur as a result of sharing personal items such as razors or toothbrushes. Therefore it is understandable that infection could also be transmitted within correctional settings.
We tested these possibilities for HBV infection by testing IgM anti-HBc (in one serum sample) and seroconversion in two serum samples. We excluded the possibility of infection outside prison before arriving in prison (incubation period) by testing >3 months after incarceration. Similarly, incubation period was excluded for acute HCV infection tested through seroconversion and increasing number of different anti-HCV by RIBA. After adequate approximation of absolute number of acute HBV and acute HCV to the yearly level, we found that HBV infection inside prison increases by 0·5% and HCV infection by 1·2%.
Our finding of 0·5% for HBV is lower than reports from the USA where incidence rates have ranged from 0·8% to 3·8% per year [Reference Weild15]. Risk of HCV acquisition during incarceration (acute HCV infection) is not well established. Our data of 1·2% is similar to a report from the USA (1·1%) [Reference Weinbaum, Sabin and Santibanez23].
These figures strongly suggest the necessity of continuous educational programmes among the prison population and the introduction of HBV vaccination.
Additional reason for such programmes results from our finding that only a very low level of the prison population is protected against HBV infection, i.e. only 4·3% in adults and 30·0% in juveniles.
Juveniles
HBV and HCV transmission inside our prisons has not been observed in juvenile correctional settings.
HIV
The prevalence of HIV in European prisons varies from <1% in England to 11% in Portugal and 12% in Estonia [Reference Testa36]. Our findings of 0·16% of HIV positivity are in accord with a report from Greece (0–0·19%) [Reference Mrani37], but in the majority of reports from different countries it is higher, ranging from 1·2% (Belgium) to 6% (France) [Reference Long8, Reference Butler13, Reference Rotily17, Reference Todts21].
Vaccination
Vaccination against hepatitis B is the best means of protecting drug users from hepatitis B and should be offered before injecting is commenced. There are only a few reports about the percentage of vaccinated prisoners at entry into prison. In a report from Denmark the percentage of already vaccinated prisoners in IDUs is very low (2·0%) [Reference Christensen38] and surveys in European prisons have found it to be 3–35% [Reference Cristensen14, Reference Testa36, Reference Weilandt and Rotily39]. Our findings show that it is low in adult prisoners generally (4·3%) and this low vaccination rate in adult prisoners entering prison is the result of a generally low response to HBV vaccination in the adult population. A better response was found in the younger population (juveniles) and, naturally, the best was in staff who were obliged to do so by regulation. A low response rate (production of antibodies to HBsAg, anti-HBs) in adults could be the result of different reasons: inadequate application of vaccine, old vaccine, inappropriate storage conditions (inadequate temperature), incorrect answer from prisoners (whether they went through complete vaccination procedure or not, i.e. all three doses or only one or two).
CONCLUSIONS
The presented results indicate that the prison population is of special interest for the testing of hepatitis markers. Of this population the majority are IDUs with a high percentage of HBV and HCV positivity. It is well known that IDUs outside prison are difficult to control. Inside prisons they can be tested for hepatitis markers, educated regarding the sense of transmission and protection of transmission of HBV and HCV, protected specifically against HBV infection (HBV vaccine) and could commence HCV and HBV therapy. Additional factors for such controls are data which show a high percentage of HBV/HCV co-infection, low level of HBV vaccination and relatively high spread of HBV and HCV infection within the prison population. Improved access to medical care and preventative services for the incarcerated population can benefit communities by reducing disease transmission and medical costs. Implementation of such programmes is of great importance not only for individuals but also for society in general. In addition, since a substantial proportion of prisoners released into to the community continue to acquire or transmit these infections at a high rate, correctional efforts should become part of the prevention and control efforts in the broader community.
Based on these results, we introduced the following programme in our prison system: systematical testing of all incoming prisoners, providing precise information on HBV/HCV infection, giving HBV vaccination and providing therapy. This programme has run continuously for almost 1 year and given excellent results.
DECLARATION OF INTEREST
None.










