INTRODUCTION
Cholera is an infectious, acute watery diarrhoea caused by Vibrio cholerae serogroups O1 and O139 [Reference Miller, Feachem and Drasar1, Reference Tauxe, Mintz and Quick2]. India remains endemic for cholera, particularly in the Eastern part [Reference Sur3]. V. cholerae O139 has been present in the country since 1992 [Reference Ramamurthy4], but does not account for the majority of cases [Reference Singh5]. In the Eastern state of Orissa, clusters of acute diarrhoea are common. However, laboratory investigations are difficult. Many clusters of diarrhoea are not investigated using laboratory methods that could lead to the identification of an aetiological agent. Thus, cholera may be under-recognized [Reference Zuckerman, Rombo and Fisch6].
In September and October 2005, the Orissa Multi Disease Surveillance System (OMDSS) captured an increase of incidence of watery diarrhoea that occurred consecutively in Chisapada and Tentulipatna, two villages located 2·5 miles apart in the Belatikiri Primary Health Centre (PHC) area, in Dhenkanal district. A comparison of the 2005 incidence with the 2003 and 2004 data pointed to unusually high rates. This increase of incidence rates took place in the absence of influx of population in any of the villages during that period and in the absence of any changes in the surveillance system. We investigated these two outbreaks to characterize the agent, describe the persons affected, identify the sources and formulate recommendations for control.
METHODS
Descriptive epidemiology
We defined a case of diarrhoea as the occurrence of more than three watery stools a day among residents of any of the two villages between 1 September and 31 October 2005. This case definition was consistent with the recommendations of the OMDSS. The primary investigator, local health workers and laboratory technicians searched actively for cases by a door-to-door search in the two villages. We collected personal history, including symptoms, from case-patients and established a line listing. We constructed an epidemic curve to describe the development of the outbreak over time. We plotted the cases on a map to understand the geographical distribution of the disease. We calculated the incidence by age and sex using population denominators collected during the door-to-door case search. In Chasapada, the first village, we were able to identify an initial small cluster in the family of a milkman. We interviewed the family to determine whether they could have constituted a source of infection for the larger outbreak. We also generated hypotheses regarding the possible modes of transmission of cholera in the two villages on the basis of the time, place and personal characteristics of the outbreak.
Matched case-control studies
To test our hypotheses regarding the possible sources of infection in the two villages, we conducted a matched case-control study in each of the two villages using similar methods. We included all cases identified during the door-to-door case search. For each case-patient, we recruited one neighbourhood control subject who was matched for sex, age (1 year) and socioeconomic status. We collected information with respect to potential exposures from subjects using a standardized, close-ended questionnaire. For instance, in Chasapada, the first village, we collected information specific to food from a hotel or consumption of milk products prepared in the index case-patient's household. In Tentulipatna, the second village, we collected information on drinking of water from the suspected well, working in houses where a patient was recovering from acute diarrhoea and eating food from a street vendor. We calculated matched odds ratio (mOR) for discordant pairs and their 95% confidence intervals (CI) using Epi-Info version 6.04d (CDC, Atlanta, GA, USA).
Laboratory procedure
We collected stool specimens from a sample of case-patients selected at random before any treatment with antibiotics. We sent those to the Regional Medical Research Centre (RMRC) of the Indian Council of Medical research (ICMR) in Bhubaneswar, the state capital. The laboratory conducted streak culture in alkaline nutrient agar for vibrio and identified the agent using biochemical methods, serum agglutination, sheep red blood cell haemolysis and serotyping methods.
Environmental investigations
In the first village, where descriptive and analytical epidemiological data suggested that the outbreak was foodborne, we reviewed food-handling practices. We interviewed the family of the milkman about hygiene practices and observed them handling milk products. In the second village, where a well was suspected as the source of the outbreak, we reviewed the water supply and sanitation situation through observation and interview of community leaders.
RESULTS
Chasapada
Descriptive epidemiology
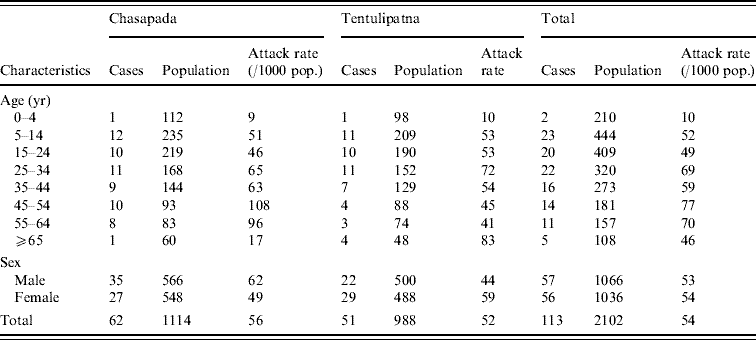
We identified 62 cases among the 1114 residents of the village (attack rate 56/1000). There were no deaths. Males were more affected than females (Table 1). Attack rates were highest in the 45–64 years age group (Table 1). Cases were scattered throughout the village in the absence of any clustering (Fig. 1). The initial case-patient, was aged 5 years, became sick on 17 September, received care from his father and uncle who became sick within 24 h. Two other members of family were affected in the following days (Fig. 2). Following this initial family cluster, there was a peak of cases on 21 September followed by a decrease in incidence, suggesting a persisting common-source outbreak. Hypothesis-generating interviews with case-patients indicated that many of them had consumed food from a small hotel in the village. In addition, case-patients reported consuming dahi (Indian yogurt) and cheese prepared by a milkman who was from the household where the initial case was reported. Thus, we generated the hypotheses that the source of the outbreak could have been food from the hotel or consumption of milk products prepared in the index case-patient's household.
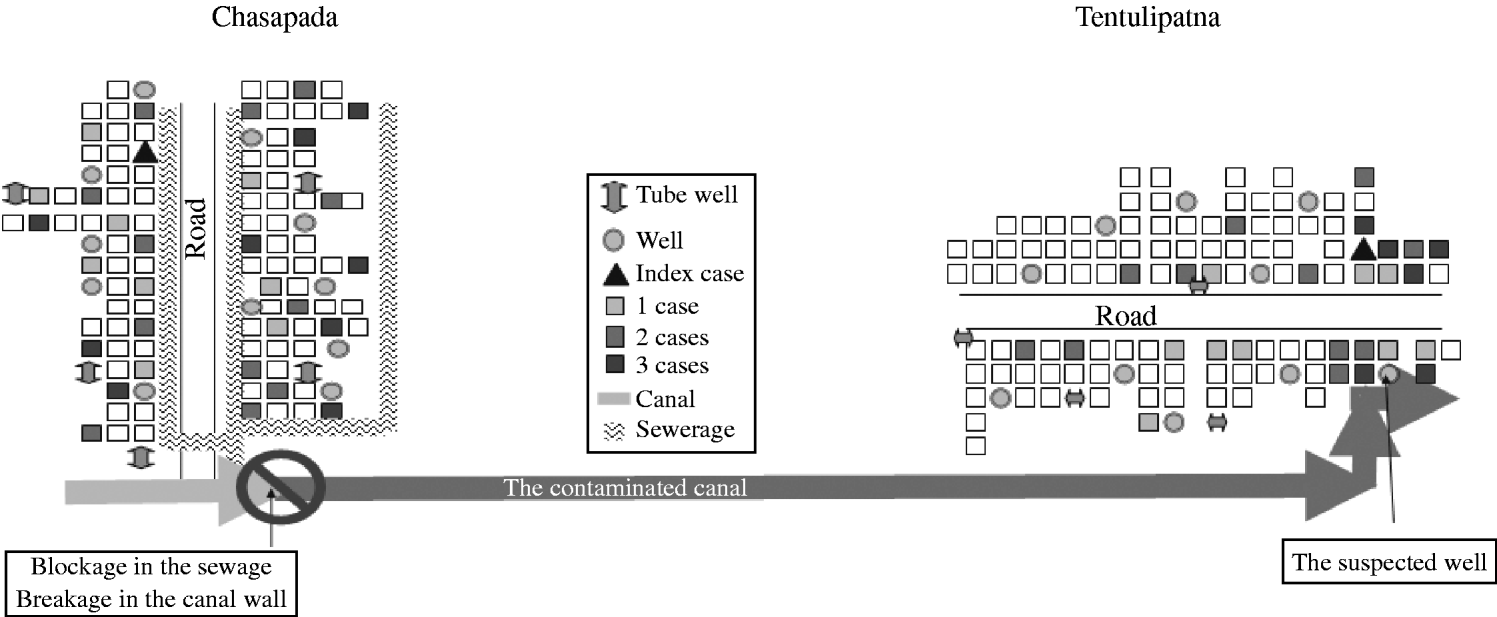
Fig. 1. Cases of acute diarrhoea by place of residence in the two villages, Orissa State, India, September–October 2005.
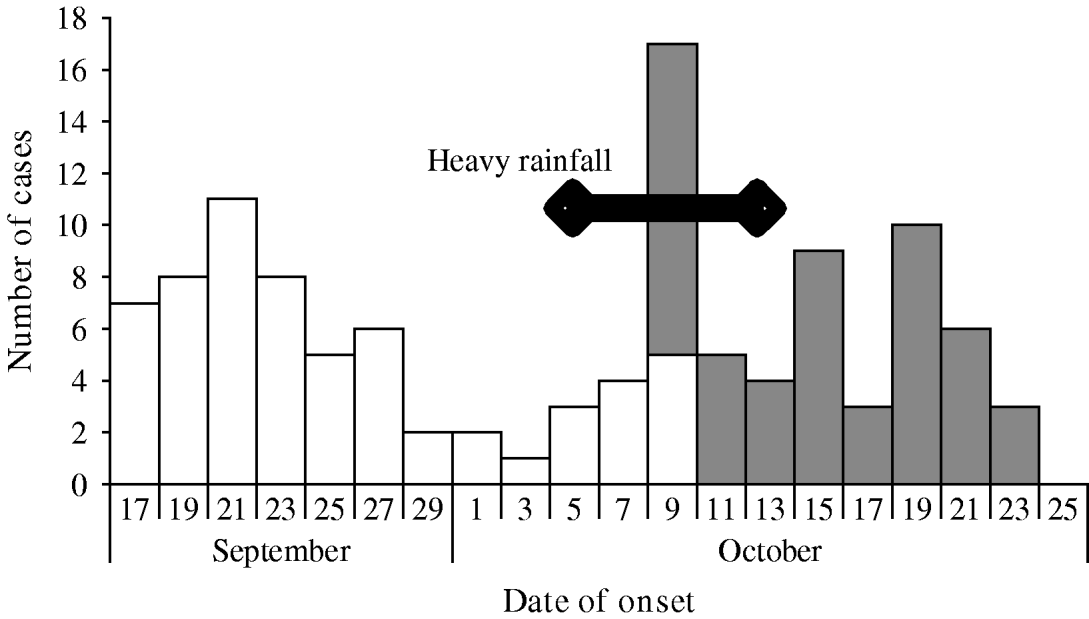
Fig. 2. Cases of acute diarrhoea by date of onset, Chasapada (□) and Tentulipatna (![]() ) villages, Orissa, India September–October 2005.
) villages, Orissa, India September–October 2005.
Table 1. Attack rate (per 1000 population) of acute diarrhoea by age and sex in Chasapada and Tentulipatna villages, Orissa, India, September–October 2005
Analytical epidemiology
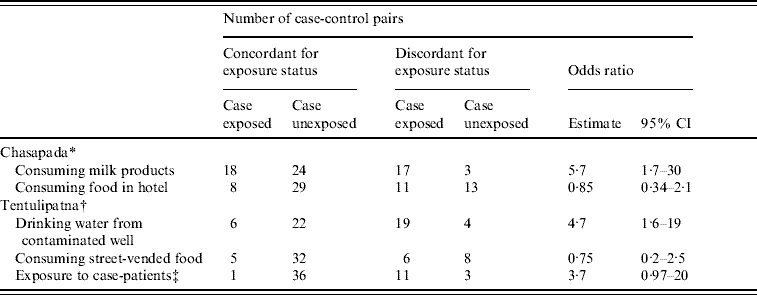
We recruited 62 matched case-control sets for the case-control study. The median age was 30 years and 29 case-patients (47%) were female. Of the cases, 57% had history of consumption of milk products prepared in the household of the index case. While 42 pairs were concordant for that exposure, 17/20 discordant pairs suggested that consumption of milk products prepared in the first case-patient's household was associated with illness (mOR 5·7, 95% CI 1·7–30; Table 2). Consumption of food prepared in the hotel was not associated with illness.
Table 2. Case-control pairs according to the exposure status of cases and controls, Chasapada and Tentulipatna villages, Orissa, India, 2005
CI, Confidence interval.
* 62 case-control pairs.
† 51 case-control pairs.
‡ Working in houses where diarrhoea case-patients were recovering.
Tentulipatna
Descriptive epidemiology
We identified 51 cases in Tentulipatna among the 988 residents (attack rate 52/1000). One death was reported (case fatality 2%). The attack rate was higher among males and among those aged ⩾65 years (Table 1). The outbreak started abruptly with an initial peak on 9 October 2005 (Fig. 2). The number of cases decreased thereafter, although a number of subsequent clusters occurred before the outbreak finally ended on 23 October 2006. In contrast to the first village where cases were scattered throughout the village, cases clustered around one well, suggesting this could have been the main source of infection. Hypotheses-generating interviews led to the identification of two other possible sources of infection that were reported by a high proportion of case-patients: working in houses where a patient was recovering from acute diarrhoea and eating food sold in the streets of the village.
Analytical epidemiology
We recruited 51 case-control sets for the case-control study. The median age was 27 years and 57% of subjects were female. Of the cases, 50% had drunk water from the suspected well. While 28 pairs were concordant for that exposure, 19/23 discordant pairs suggested that drinking well water was associated with illness (mOR 4·7, 95% CI 1·6–19; Table 2). Working in a house where a patient was recovering from diarrhoea was associated with illness although this was not statistically significant. There was no association between consumption of food items from the street vendor and illness.
Laboratory investigations
We collected seven rectal swabs from patients who had not consumed antibiotics. Of these, four could not reach the laboratory. Out of three stool samples sent for culture, two (one from each village) were positive for V. cholerae El Tor O1 Ogawa.
Environmental investigations
The milkman's family reared cows. He sold raw milk, dahi, cheese, buttermilk and butter to the villagers. He did not wash his hands as he perceived that it could alter milk products. He added water to the milk (adulteration). Cleaning was insufficient and storage of utensils was unhygienic, allowing cross-contamination between clean and dirty utensils. There were multiple opportunities for the contamination of the milk products by dirty hands. Milk products were stored at room temperature.
The time sequence between the two outbreaks suggested that the second outbreak might have been a consequence of the first. A sewerage drain collecting the wastewater from Chasapada village (the first village) ran parallel to a road (Fig. 1). That drain did not have a proper terminal disposal system and ended in close proximity to an irrigation canal that flowed towards Tentulipatna, the second village. On 6 October, roadwork labourers blocked the drain and destroyed the embankment of the canal for renovation of the nearby road, causing the sewerage water to accumulate. Heavy rainfall between 6 and 13 October caused the drain to overflow. It then contaminated the canal that flowed towards Tentulipatna located 5 km away. In the second village, the canal passed by the implicated well from which it was only 2 m apart. That well was unprotected and did not have any brim or platform.
DISCUSSION
These two outbreaks of cholera were related. The first was mostly foodborne and secondary to the consumption of milk products prepared in the household of the index case-patient. The second was mostly waterborne and caused by drinking water from a well that had been contaminated by sewerage originating from the first village. These two outbreaks illustrate the various modes of transmission of cholera in the Indian state of Orissa. They also point to potential prevention measures.
Foodborne transmission of cholera is less common than waterborne transmission [Reference Shaffer, Mendes and Costa7, Reference St Louis8]. While water may be the primary vehicle of transmission in endemic areas, as the disease spreads within the community, secondary transmission via food items, food handlers and from person-to-person contact increases [Reference Miller, Feachem and Drasar9]. Food items initially free from V. cholerae may become contaminated when mixed with water, mixed with other contaminated food or handled by infected persons who have not observed proper hygiene procedures. The milk handlers of the milkman's family added water to the milk and to the dahi before sale. Hand washing was unlikely to have been consistent as they reported it could alter milk products. Soap is inexpensive and accessible. Hand washing with soap is effective in preventing diarrhoeal diseases [Reference Shahid10]. Failure to wash hand with soap has been reported as a risk factor for cholera [Reference Hutin, Luby and Paquet11]. However, in the case of food handlers, the public health benefit of hand washing with soap extends well beyond the person who is actually washing hands to include consumers. If the members of the milkman's household had (1) refrained from handling food while they had diarrhoea, (2) washed hands with soap, (3) maintained a cold chain, and (4) abstained from adulteration of the milk, almost half of the cases of cholera that occurred in the first village might have been prevented.
Contaminated water is often reported as the source of infection during cholera outbreaks in India [Reference Hamner12, Reference Sur13]. During the outbreak in the second village, half of the cases drank water from an unsafe, unprotected well that had been contaminated with sewerage water originating from the first village. In fact, a third outbreak of 18 cases occurred in another village due to use of stream water for bathing and washing utensils, which was also contaminated by the contaminated canal water. We did not include this third outbreak in this report because of excessive limitations in the documentation (e.g. no laboratory confirmation). Scholars from the Indian Field Epidemiology Training Programme investigated three cholera outbreaks in three states between 2001 and 2005 [Reference Das, Rao and Gupte14–Reference Saha, Ramakrishnan and Gupte16]. Of these, all were associated with unsafe water sources. In 2003, we investigated an outbreak of cholera in the same district of Orissa. This outbreak had been caused by an unprotected well that had been contaminated by an index case-patient [Reference Das, Rao and Gupte14]. Following that investigation in 2003, the well that caused the outbreak in the village was protected. A total of 210 privately dug wells constructed since 2003 in the district were built with protection. In addition, the Government constructed 518 new, safe tube wells. Overall, in 2006, and despite these efforts in the district, about 30% of the wells were still unprotected. Thus, there is a need for continued efforts to protect the backlog of unsafe, unprotected, old wells.
Our investigation had two main limitations. First, we were not able to obtain laboratory confirmation for more than one case in each village because of prior antibiotic use, refusals and logistical difficulties. Thus, we cannot exclude that the two outbreaks could have been caused by a combination of microorganisms. However, the frequency of symptoms among case-patients, the severity of the diarrhoea and the case-fatality ratio were compatible with the diagnosis of cholera. Furthermore, the conclusion of our investigation and our recommendations would not be different if the agent had been different (e.g. Norovirus). Second, in both villages, none of the two sources identified accounted for the majority of cases. In the second village, a number of cases may have been secondary to person-to-person transmission, including through persons working in households where case-patients were recovering. During cholera outbreaks, more than one source is commonly involved. Environmental contamination is substantial because of the high concentration of the pathogens in the stool of patients. However, the common sources of infection that we identified in each village constituted an initial input to the outbreaks that was probably essential to their dynamics even though these sources might not have accounted for the majority of cases.
These two cholera outbreaks were related although they were transmitted through different vehicles. Mishandling of milk products led a large number of cases in the first village, which led to sewerage contamination of a well and a second outbreak in the next village. On the basis of our conclusions, we formulated a number of recommendations and engaged in a number of interventions. In the first village, we educated the milkman's family. Specifically, we advised them not to prepare milk products until the outbreak was over. We also provided guidance on hygienic practices addressing the issues identified during our environmental investigation, with a specific focus on abstaining from adulteration of milk. In the second village, local health personnel had chlorinated all wells heavily before the results of the investigation. However, on the basis of our results, we held a meeting and advised the public not to use the incriminated well. We advised the owner of the well to construct a brim and to repair the inner side that had allowed the canal water to enter. We advised the public not to use household servants until the outbreak was over. We debriefed the results of the investigation with the contractor who was renovating the road so that he opened the drain immediately. We had discussion with the local engineer regarding rural water sources and sanitation and requested a proper disposal system for the sewerage drain from the first village. On the basis of our discussion, they changed the path of the drain to a distant unused forest, where the sewerage will dry up. Overall, these two outbreaks illustrate how cholera might spread from village to village in endemic areas. Environmental contaminations should be expected and prevented during all cholera outbreaks. To control cholera in the longer term, safe food-handling programmes are needed, as well as safer, protected wells and adequate sanitation. Further, investigations of diarrhoea clusters must be systematic and careful as it cannot be taken for granted that all cholera outbreaks in India are waterborne.
DECLARATION OF INTEREST
None.






