INTRODUCTION
The sheep tick Ixodes ricinus is the main vector involved in the transmission of the Western European subtype of tick-borne encephalitis virus (TBEv) in north-eastern Italy, as well as in other European countries where foci have been detected [Reference Gritsun, Lashkevich and Gould1, Reference Süss2].
In the last few decades, an overall increase in the incidence of TBE in Central Europe and the Baltic States has been recorded [Reference Randolph3]. In particular, in the early 1990s the incidence of TBE considerably increased within a few years in the Baltic region, whereas elsewhere, e.g. Germany, Slovakia, Switzerland and Czech Republic, the number of human cases has risen more gradually, and to a lesser extent [Reference Randolph4, Reference Sumilo5]. In Italy, the first human cases were reported in Tuscany in 1975 [Reference Amaducci6]. Although TBE is of marginal medical importance in Italy compared to the Baltic and Nordic countries, a tenfold growth in the incidence of TBE was recorded from 1975 to 2001 [Reference Süss2]. Currently, most human cases of TBE in Italy are confined to the north-eastern region of the country [Reference Beltrame7]. The TBE incidence rate recorded in the province of Trento, where the present study was undertaken, was estimated to be about 0·60/100 000 inhabitants per year for the period 1992–2004 [Reference Rizzoli8].
The upsurge of TBE is probably the result of several factors. It may be explained partly by improvements in qualitative and quantitative epidemiological surveillance systems and diagnostics [Reference Rendi-Wagner9]. On the other hand, this increase has been related also to an intensified exposure of humans to ticks and, in specific countries, by social and political changes, e.g. increased forest frequentation for resource exploitation or leisure [Reference Rogers and Randolph10, Reference Sumilo11]. In turn, the tick cycle depends both on climatic variables and host availability, which can condition tick abundance and spatial distribution [Reference Lindgren and Gustafson12–Reference Daniel14].
Viraemic transmission alone did not seem to explain TBE prevalence and distribution [Reference Randolph15]. Several studies showed experimentally that transmission of TBE between immature stages of ticks is mediated through non-viraemic infection [Reference Labuda16] which occur at the local skin site where ticks feed [Reference Gritsun, Lashkevich and Gould1, Reference Labuda17]. Therefore, a particular pattern of tick seasonal dynamics influences TBE persistence, such as the synchrony of feeding larvae and nymphs on rodent hosts, especially the yellow-necked mouse (Apodemus flavicollis) [Reference Randolph15, 18–Reference Labuda20]. This pattern has been statistically associated with a high rate of autumnal temperature decrease relative to the annual maximum of the monthly mean temperature (‘autumnal cooling rate’, sensu Randolph et al. [Reference Randolph18]). This condition explains why TBE is characterized by a high spatio-temporal heterogeneity as it occurs only in discrete foci within the tick's distribution [Reference Rogers and Randolph10] in contrast to Lyme Borreliosis, which occurs wherever competent tick species exist. Climate also indirectly influences the prevalence of TBEv in ticks by modulating population size and distribution of several host species [Reference Lindgren and Gustafson12], together with human-driven factors, such as changing agricultural or game management practices [Reference Hudson21].
Although ungulates do not appear to be susceptible to TBEv and do not support viraemia [Reference Randolph15, Reference Labuda20, Reference Labuda22], they play a considerable role in the ecology of TBE by feeding the adult stage of I. ricinus, thus modulating local tick abundance [Reference Hudson21, Reference Perkins23]. Among the ungulates, deer, and particularly roe deer Capreolus capreolus are the most abundant wild hosts in Europe, common in deciduous woods that are potentially suitable habitats for ticks [Reference Geist24]. There is also evidence that deer can support a high proportion of immature stages of ticks, larvae and nymphs [Reference Matuschka25]. Previously, roe deer have been used as sentinels for endemicity of TBEv and TBE-complex virus in some sites in Germany and Denmark, respectively, using serology [Reference Süss26–Reference Skarphédinsson, Jensen and Kristiansen28]. In the eastern Italian Alps, where the province of Trento is located, roe deer are the principal wild ungulate in terms of abundance and distribution and have continuously increased over the past decades from about 12 000–30 000 (Autonomous Province of Trento, Game Management Assessment, 2003). Previous studies have evaluated tick occurrence on this species in relation to ecological variables and have found vegetation and altitude as strong predictors of intensity [Reference Chemini29]. The abundance of questing ticks and ticks feeding on roe deer has been found to be greater in TBE-positive hunting districts (human cases) than in hunting districts where TBE has not been recorded [Reference Hudson21]. A significant correlation between roe deer abundance (number of harvested roe deer) and TBE incidence was also found in the Czech Republic [Reference Zeman and Benes13].
In this study, we used roe deer as sentinels of tick abundance from a regional to local scale, in relation to geographic and climatic factors that have already been related to TBE emergence and persistence. Therefore, the aims of this study included:
• to verify the relation between roe deer tick infestation and geographic factors (hunting districts, altitude);
• to assess the relation between roe deer tick infestation and climatic factors;
• to test the association between tick infestation, geographic and climatic parameters and the occurrence of TBE human cases.
METHODS
Study area
The study was undertaken in the Province of Trento, north-eastern Italian Alps, in 19 hunting reserves, selected across the whole geographic and altitudinal range. The game reserves cover an area of 69 210 km2 where the altitude ranges between 150 m and 2050 m a.s.l. (centroid coordinates 11·21 E, 46·10 N) and 55% of the territory is covered by coniferous and deciduous forest. Of the investigated areas, most of the human clinical cases of TBE recorded in Trentino were from the Northern Sarca hunting district.
Tick collections from deer legs
The study was carried out on 132 roe deer shot during the first 2 weeks of September 2004, at the beginning of the hunting season. At this time of the year, roe deer are still territorial, thus almost evenly distributed in the area and representative of a certain geographic context [Reference Hewison, Vincent, Reby, Andersen, Duncan and Linnell30]. To assess the pattern of tick infestation on roe deer, the lower part of the forelegs (distal to carpal joint) was removed immediately after the roe deer was shot and sealed in a plastic bag. Site and altitude were recorded for each sample. The samples were stored at −20°C before processing and then examined for tick abundance and presence of coincidental feeding of larvae and nymphs (adults unconsidered) [Reference Gilot31]. In particular, we define co-feeding as an aggregation of any tick life-stage (without distinguishing between larval-nymphal and nymphal-nymphal aggregations and excluding larvae-larvae associations as these are likely to be pathogen free), feeding at a perceptible distance (about 1 cm, following Randolph et al. [Reference Randolph, Gern and Nuttall19]).
Ticks were counted and examined microscopically to determine species and stage using a reference key [Reference Manilla32].
Climatic and geographic data
Following the methodology from Randolph et al. [Reference Randolph18], we calculated the autumnal cooling rate (decrease of autumnal temperature relative to the annual maximum of monthly mean temperature) from satellite data. We obtained a time-series of MODIS Land Surface Temperature (LST) data (level V004) through the NASA EOS Data Gateway. For our study, we used MODIS data for 2003 since we analysed the pattern of tick infestation in 2004, which, in turn, is the result of tick development in 2003 [Reference Randolph18]. We processed data from the Terra (T) and Aqua (A) satellites [to gain overpasses four times a day, at about 01:30 hours (A), 10:30 hours (T), 13:30 hours (A), and 22:30 hours (T), local time]. The original LST data are provided in Kelvins at a pixel resolution of 1 km2, in sinusoidal projection. We applied a pre-processing procedure, which included a re-projection to the Italian Gauss-Boaga cartographic system, the application of the bit-pattern encoded MODIS quality assurance (QA) maps to remove poor-quality pixels and to convert Kelvins to degrees Celsius (°C) [Reference Rizzoli8, Reference Neteler33]. To calculate the autumnal cooling, we applied a linear regression to the monthly LST mean values of August, September and October time-series. In addition, we selected the annual maximum of the monthly mean LST values per pixel. We examined the cooling rate in relation to the maximum LST value as they are positively correlated.
Further GIS (Geographical Information System) data were prepared: hunting reserves were intersected with elevation belts (0–499 m, 500–999 m and ⩾1000 m a.s.l.). For the resulting subdivided hunting district polygons, we extracted autumnal cooling data statistics, and particularly: autumnal decrease in temperature, annual maximum of monthly mean temperature and autumnal decrease divided by annual maximum of monthly mean temperature.
The entire procedure was implemented in GRASS GIS 6 and R statistical language [34, Reference Neteler and Mitasova35].
Statistical analysis
We investigated if the pattern of tick infestation on roe deer, and in particular the number of co-feeding ticks, and the occurrence of TBE human cases (1992–2004) were predicted by geographical and climatic parameters. We therefore fitted a generalized linear model (GLM) with either negative binomial error distribution (for the response variables N_Ticks, nymphs and larvae, N_Nymphs nymphs only and Co-feeding, number of co-feeding ticks) or binomial error distribution (for the response variable TBE, occurrence of TBE human cases in hunting districts) by means of R statistical language [34] using the R package MASS [36]. We tested the effects of altitude (ALT), location (DIST: 19 hunting reserves pooled in seven hunting districts) on N_Ticks, N_Nymphs, Co-feeding and TBE. On the same response variables, we also tested the effect of the annual maximum of monthly mean temperature (T max), autumnal decrease of temperature (Cool) and the rate among these two parameters (Cool/T max). These two variables were entered into the model with a negative sign.
Even though the autumnal cooling rate was originally related to spring tick activity [Reference Randolph18], later analyses on tick seasonal dynamics point out the emergence of a single cohort of each stage of ticks each year in the autumn [Reference Randolph37]. We based our analyses on this observation.
Moreover, we tested whether the TBE cases can be predicted by tick infestation fitting a GLM with binomial error distribution.
Response variables were modelled for dependence on predictor variables using the model selection method based on the Akaike information criterion (AIC) corrected for small sample size, AICc [Reference Burnham and Anderson38]. From the AICc differences (ΔAICc), we calculated AICc weights (ω) and the relative evidence ratios. When differences in AICc values were <2, indicating approximately equal parsimony of models, we ranked all variables considered in the full model according to their importance [predictor weights ω+(j)]. Parameter estimates were evaluated for the best model or by model averaging, in case of equal parsimonious models, weighting estimates from each model according to their Akaike weight.
RESULTS
Out of 132 roe deer legs analysed, 77·3% were infested with at least one tick. The total ticks counted were very high, with a total of 4754 larvae, 630 nymphs and 15 adults. I. ricinus was the only tick species recorded. The adults were not considered in the statistical analysis, due to the small sample size. The pattern of tick infestation on roe deer samples showed a typical aggregated distribution of larval and nymphal ticks. The mean number of larvae and nymphs were 36·02 (s.e.=5·20) and 4·78 (s.e.=0·74), respectively. The highest observed infestation for one sample was 388 ticks (both nymphs and larvae).
The maximum of mean monthly temperature recorded in 2003 in the hunting districts where roe deer samples were collected varied between 16·84°C and 25·21°C, while the decrease of temperature from August to October ranged between −8·05°C and −6·13°C.
In the present study, we found significant differences in tick infestation on roe deer along the geographic range. The best model explaining total number of ticks included altitude, hunting district and the interaction between those two variables (Table 1a). As shown by the best model coefficients, tick infestation level was significantly higher in some geographic contexts (i.e. hunting districts and hunting district∗altitude) than in others, while altitude alone did not exert any significant effect (Fig. 1, Table 3). We obtained similar results for number of nymphs only (Tables 1a, 3). The best model to explain number of co-feeding ticks with respect to geographic attributes included altitude and hunting districts (but not the interaction between them; Table 1a, Fig. 2). Specifically, model coefficients showed a significant negative correlation with altitude and an uneven number of co-feeding ticks within hunting districts (Table 3). TBE human cases were predicted by hunting districts only, although coefficients were not properly estimated (Tables 2b, 3).

Fig. 1. Mean number of larvae (■) and nymphs (□) per roe deer sample in relation to the geographic location (hunting districts). * Indicates the hunting district where tick-borne encephalitis human cases were recorded since 1992.
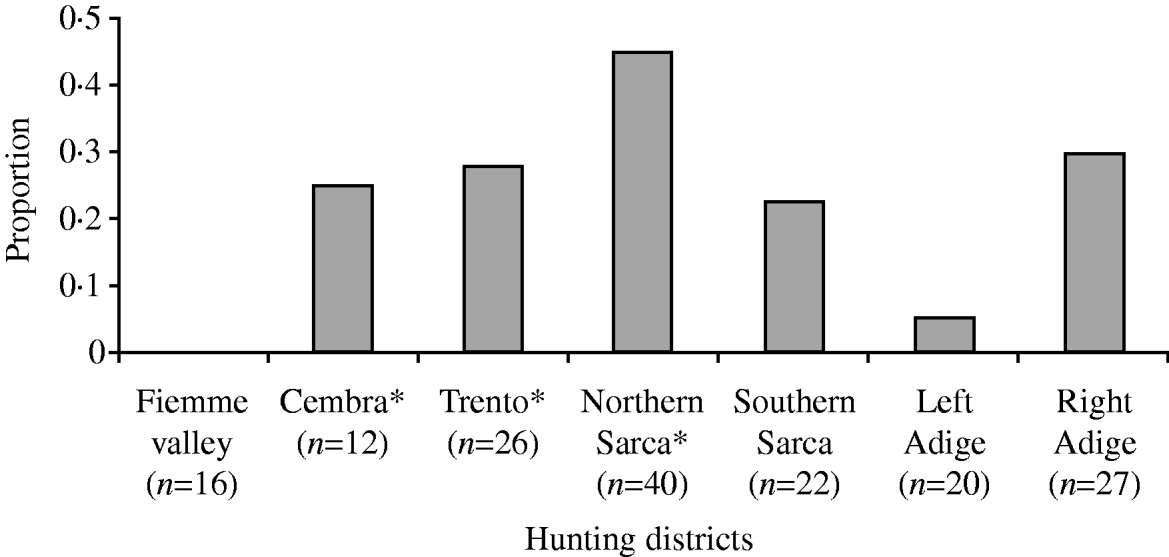
Fig. 2. Proportion of roe deer samples with co-feeding in relation to geographic location (hunting districts). * Indicates the hunting district where tick-borne encephalitis human cases were recorded since 1992.
Table 1. Akaike's Information Criterion (AICc) ranking of a priori models to estimate dependence of total number of ticks, nymphs and co-feeding on roe deer samples from (a) geographic and (b) climatic parameters, respectively

K, Number of parameters; ΔAICc, difference in AICc between the best and the actual model; ωi, Akaike's weight; Evidence ratios, ratio of Akaike's weights of the best and the actual model. The table shows only the first two ranked best models.
† N_Ticks, number of total ticks (larvae and nymphs) counted on roe deer legs; N_Nymphs, number of nymphs counted on roe deer legs; Co-feeding, number of co-feeding ticks on roe deer legs; ALT, altitude; DIST, hunting districts; T max, annual maximum of the monthly mean temperature for 2003; Cool, autumnal temperature decline recorded during 2003; Cool/T max, rate of autumnal temperature decrease relative to the annual maximum of the monthly mean temperature for 2003.
Table 2. Akaike's Information Criterion (AICc) ranking of a priori models to estimate dependence of tick-borne encephalitis (TBE) human cases from (a) tick burden, (b) geographic and (c) climatic parameters, respectively
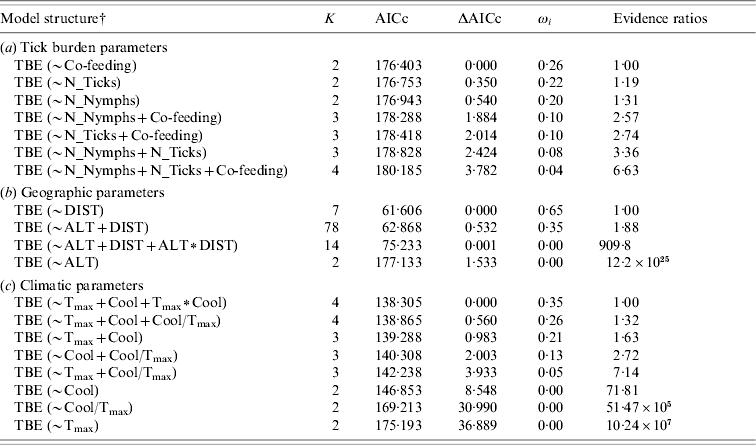
K, Number of parameters; ΔAICc, difference in AICc between the best and the actual model; ωi, Akaike's weight; Evidence ratios, ratio of Akaike's weights of the best and the actual model.
For parameter abbreviations see Table 1.
† TBE, Sites where TBE human cases were recorded.
Table 3. Parameter estimates relative to the best model (ΔAICc >2) or evaluated by model averaging, in case of equal parsimonious models, for the response variables total number of ticks, nymphs only, number of co-feeding on roe deer samples and tick-borne encephalitis (TBE) human cases. Theta (θ) refers to the dispersion parameter of the selected model when a negative binomial error distribution is applied
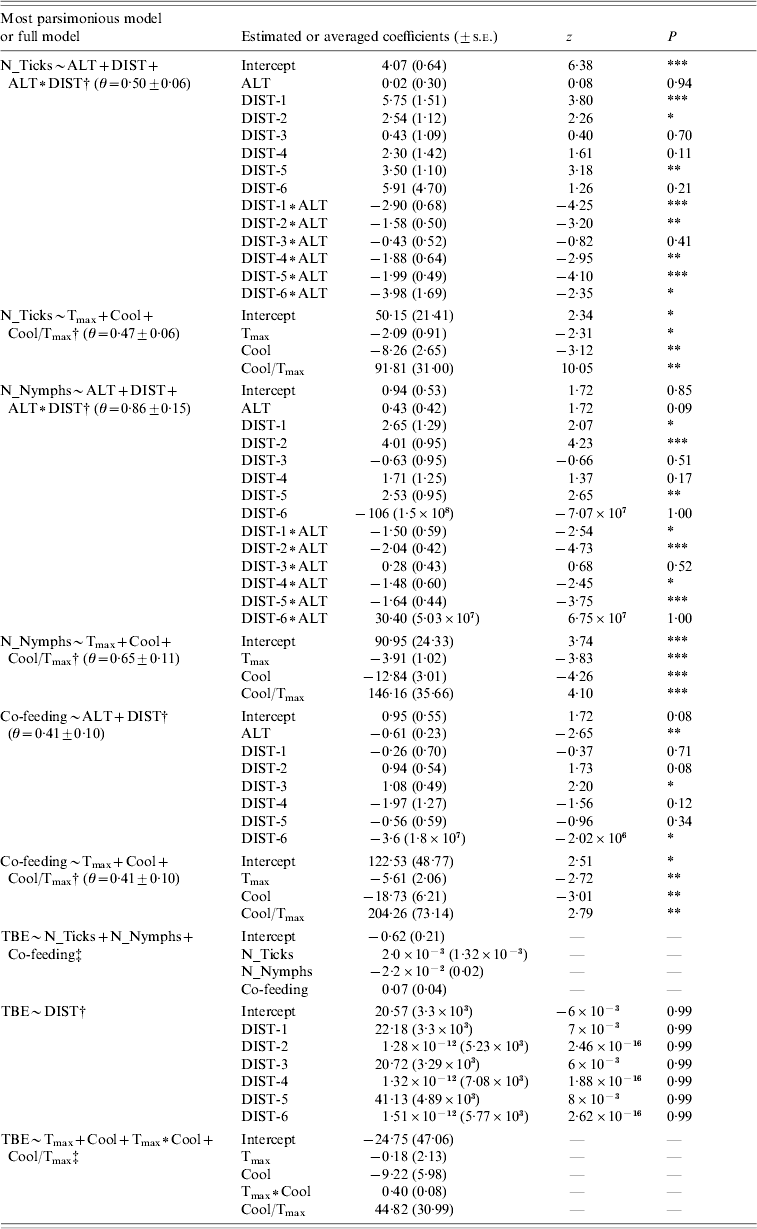
† Best model
‡ Full model.
DIST, Hunting districts; DIST-1, Cembra; DIST-2, Right Adige; DIST-3, Northern Sarca; DIST-4, Left Adige; DIST-5, Trento; DIST-6, Fiemme Valley.
For parameter abbreviations see Table 1.
* P⩽0·05; ** P⩽0·01; *** P⩽0·001.
Total number of ticks, nymphs only and number of co-feeding ticks were also significantly affected by climatic factors (Table 1b). Specifically, there was a weak negative correlation with both the annual maximum of monthly mean temperature and the autumnal temperature decrease, whilst the rate among these two variables had a strong positive effect. All climatic factors were relevant predictor variables for TBE human cases (predictor weights: T max=1·00, Cool=0·95, T max∗Cool=0·35, Cool/T max=0·44) and included in either equal parsimonious model (Table 2c), but only the rate of the autumnal temperature decrease to the annual maximum of monthly mean temperature had a strong positive effect, as shown by the model averaged coefficients (Table 3).
Tick infestation variables were all relevant predictor variables for TBE human cases (predictor weights: N_Ticks=0·43, N_Nymphs=0·42, Co-feeding=0·50; Table 2a), with the number of co-feeding ticks having a stronger effect than the total number of ticks and nymphs only (Table 3).
DISCUSSION
In the present study, we investigated the pattern of tick infestation on roe deer in relation to geographical and climatic parameters in the province of Trento, north-eastern Italian Alps. At a regional scale, we found that tick abundance on roe deer was clearly associated with the geographic location, whereas altitude alone scarcely explained this variable. This result is consistent with the strong effect of autumnal cooling rate [Reference Randolph18] on tick abundance that we recorded, because the autumnal temperature decrease relative to the annual maximum of the monthly mean temperature is a climatic profile which is likely to be site specific in a discontinuous and patchy environment such as the Alpine range. Here, the altitudinal variation and geomorphological features (e.g. valleys, ridges and rocky outcrops) determine local micro-climates.
In roe deer samples, we frequently detected larvae and nymphs attached close together to the tissue. This association has been already defined as coincidental feeding of larvae and nymphs (co-feeding) for other species, such as small mammals [Reference Randolph15–Reference Labuda17], and it is a required condition for TBE persistence in the environment. Randolph et al. [Reference Randolph18] emphasized that presence of co-feeding ticks on hosts depends on seasonal synchrony of these different tick development stages, which is strictly associated with certain climatic conditions. In particular, the synchrony of nymphs and larvae would occur in a certain range of autumnal cooling rate, as defined above. Currently, the presence of co-feeding ticks on ruminants had not been assessed as there is no evidence that ungulates are competent hosts for TBE [Reference Labuda22]. Our study showed that, notwithstanding the indirect role of roe deer in TBE transmission, they could provide surveillance of the seasonal synchrony of immature ticks at a regional scale. In our study, co-feeding abundance on roe deer samples varied spatially and was strongly positively correlated with the autumnal cooling rate. In the hunting district where co-feeding ticks were detected in the largest proportion of samples (Northern Sarca), the synchrony between feeding larvae and nymphs had also been observed on rodents for three consecutive years, including 2004 [Reference Rosà39]. Several studies underline the importance of the peak of synchrony in spring time to the effective amplification of TBEv [Reference Labuda20]; other studies emphasize that the maximum incidence of human TBE cases coincides with the seasonal peaks of the feeding activity of I. ricinus which occur in spring and autumn time [Reference Gritsun, Lashkevich and Gould1]. In our study, samples were collected in September (hunting season) and we may, therefore, have detected the second peak of the synchrony phenomenon.
Our results suggest a geographic association between TBE occurrence in humans and tick infestation on deer, particularly abundance of co-feeding ticks [Reference Randolph18, Reference Hudson21], that in turn results in a strong positive effect of autumnal cooling rate. Consistently, the occurrence of TBE human cases was well predicted by the geographic location, in accordance with the observed heterogeneous distribution in foci of this disease [Reference Rogers and Randolph10].
In an Alpine area such as Trentino province, mainly covered by forests and characterized by an increasing intersection of natural and human exploited areas, an abundant and ubiquitous wild ungulate species, such as the roe deer, represents an ideal sentinel of TBE distribution. Collection of samples is inexpensive, being a hunted species, and sampling could easily fulfil requirements of randomness and geographic continuity. Recently, domestic ungulates (goats) were proposed as indicators of TBEv circulation in Trentino through seroprevalence assessment, together with collection of remotely sensed climatic data [Reference Rizzoli8]. Roe deer have also been used as a sentinel for endemicity of TBEv using serological investigation in several sites in Europe [Reference Labuda22, Reference Süss26, Reference Gerth27].
We suggest that monitoring tick infestation of deer, together with collection of climatic data is a potentially useful tool for verifying the occurrence of TBE-favourable conditions at a regional scale, i.e. tick abundance and synchrony of immature stages. Indeed, deer density has been shown to affect TBE persistence and circulation [Reference Randolph, Gern and Nuttall19, Reference Perkins23] together with abundance of other key hosts such as small mammals [Reference Rosà, Morand, Krasnov and Poulin40] which are more difficult to monitor, but present in similar habitats to those optimal for roe deer. Moreover, in the Alpine context, spatial distribution and movements of a crucial and mobile tick host such as roe deer is likely to affect the spatial dynamics and pattern of TBE. As possible evidence, the average home range size of roe deer in our study area [64 ha for resident individuals and 283 ha for migrant individuals (F. Cagnacci, unpublished data)], is a spatial scale comparable to that of TBE foci in Italy [Reference Hudson21]. This first screening of potential areas of TBEv circulation could then be followed by more targeted virological investigation on feeding ticks and serological investigation on competent hosts (i.e. rodents) and, for TBE risk assessment in humans, trigger considerations for leisure and routine activities taking place in high-risk areas.
ACKNOWLEDGEMENTS
This research was partially supported by the Research Fund of the Autonomous Province of Trento (Grant no. 3479 to F.C.: BECOCERWI – Behavioural Ecology of Cervids in Relation to Wildlife Infections). This research was partially funded by EU grant GOCE-2003-010284 EDEN and the paper is catalogued by the EDEN Steering Committee as EDEN 0068 (http://www.eden-fp6project.net/). We thank the Hunting Association of Trento Province (Associazione Cacciatori della Provincia di Trento) for collecting roe deer samples. Fausta Rosso is acknowledged for helping with parasite identification. We are grateful to Sarah Perkins and two anonymous reviewers who provided helpful comments on an earlier version of the paper.
DECLARATION OF INTEREST
None.







