Introduction
The high loss of faunal diversity in national parks and a growing number of endangered wildlife species have highlighted the need for an improved wildlife disease surveillance in order to identify prevalent diseases for prevention and control [Reference Belant and Deese1, Reference Grogan2]. Enhanced and timely wildlife disease surveillance and subsequent control and prevention of some of these diseases would also prevent spill over of endemic and emerging diseases to livestock and humans, supporting global health security [Reference Belant and Deese1, Reference Katz3]. However, wildlife surveillance presents unique challenges associated with limited access to animals, case identification and ecological characteristics of national parks and other forested areas where wildlife inhabit, such as expansiveness of conservation areas [Reference Ryser-Degiorgis4]. In sub-Saharan Africa, wildlife disease surveillance systems are limited to responding to large outbreaks or narrowly focused retrospective and prospective studies, approaches that have helped in implementing disease outbreak control strategies, estimating specific disease burden and identifying wildlife reservoirs of livestock or human diseases [Reference Leendertz5–Reference Hoffmann8].
Anthrax, a highly infectious disease of domestic and wild mammals caused by Bacillus anthracis bacteria, can result in a large-scale loss of wildlife and livestock worldwide [Reference Leendertz5–Reference Hoffmann8]. For example, an anthrax outbreak in Luangwa river valley in Zambia resulted in the death of >4000 hippopotami in addition to buffalos and elephants [Reference Turnbull6]. Another outbreak in Malilangwe wildlife reserve in Zimbabwe resulted in the death of almost all the kudu, and 40–70% of bushbucks, waterbucks, nyala, buffalos and roan antelopes [Reference Clegg7]. Between October 2001 and June 2002, an anthrax outbreak in the tropical forest of Ivory Coast resulted in the death of eight chimpanzees [Reference Leendertz5]. In fact, a recent comprehensive study of this tropical rain forest found that B. anthracis has been responsible for widespread and persistent mortality among domestic and wild mammals for three decades [Reference Hoffmann8]. Even in developed countries such as Canada, substantial death of wild bison associated with anthrax outbreaks has been reported [Reference Salb9]. The East African region consists of one of the largest and most diverse wildlife ecosystems in the world, in particular the contiguous ecosystem of Masai Mara and Amboseli national parks in Kenya, and Serengeti and Kilimanjaro national parks in Tanzania [Reference Okello10]. Furthermore, the world-famous annual wildebeest (Connochaetes taurinus) migration occurs between the Masai Mara and Serengeti national parks [Reference Hopcraft, Sinclair, Metzger, Mduma and Fryxell11]. A recent study in Kenya reported three anthrax outbreaks over a period of 4 years in the same locality of Rift valley in Kenya that resulted in the death of 10.5% of all wildlife herbivores in a local national park, including 17% of the buffalos and 16% endangered black rhinos and white rhinos [Reference Muturi12].
Here, we reviewed wildlife records to describe the temporal and spatial distribution of anthrax outbreaks among Kenyan wildlife between 1999 and 2017, a period when there was an improved collection of wildlife disease data in the country. This improvement was catalysed by establishing the One Health office in Kenya of which the Kenya Wildlife Services (KWS) participated in its creation since 2011. The office is operationalised through a zoonotic disease unit (ZDU) with staff drawn from Kenya's Ministries of Health and that of Agriculture, Livestock and Fisheries [Reference Mbabu13]. Since its operational launch on 1 March 2012, the need for effective wildlife health surveillance has been progressively recognised within KWS to align with ZDU's objective of instituting and maintaining active collaboration at the animal, human and environment nexus focused on better prevention and control of zoonotic diseases.
Methods
We reviewed records at the KWS of diagnosed anthrax outbreaks and occurrences in wildlife in Kenya from 1999 to 2017. Records included annual reports, specific case reports, surveillance reports and post-mortem reports. At the KWS, the anthrax disease records were generated from clinical surveillance of dead or visibly sick animals by the wildlife veterinarians and game rangers. A confirmed anthrax outbreak was defined as one in which at least one case was diagnosed using Gram and methylene blue stains and identification of the capsule and typical rod-shaped B. anthracis in clinical specimens submitted to the KWS laboratory in Nairobi or one of the regional veterinary investigation laboratories [14].
We developed a database of all the outbreaks in an MS Excel® sheet with the following as variables: conservation region, conservation area, agro-ecological zone (AEZ), month and year of occurrence, wildlife species affected and its food niche. The KWS has administratively divided the country into eight wildlife conservation regions depending on geographical location in Kenya: Coast, Tsavo, Southern, Central Rift, Western, Mountain, Eastern and Northern conservation regions (Fig. 1). Within the eight conservation regions are 60 conservation areas consisting of parks, reserves, sanctuaries and conservancies that contain wildlife that are either in protected areas (secured and monitored national parks, national reserves or national sanctuaries) or unprotected free-ranging areas where they sometimes mingle with domestic animals. AEZs are land units defined based on the patterns of soil, landform and climatic characteristics. Kenya has seven AEZs that include agro-alpine, high potential, medium potential, semi-arid, arid, very-arid and desert. Cross-tabulation of variables was carried out using Pivot tables in Excel and analysis was carried out using the Stata statistical software (Stata12/SE, StataCorp, College Station, TX, USA). We used quantum geographical information system (QGIS) software to create a map showing locations of conservation areas and free-ranging areas for Kenyan wildlife [15].
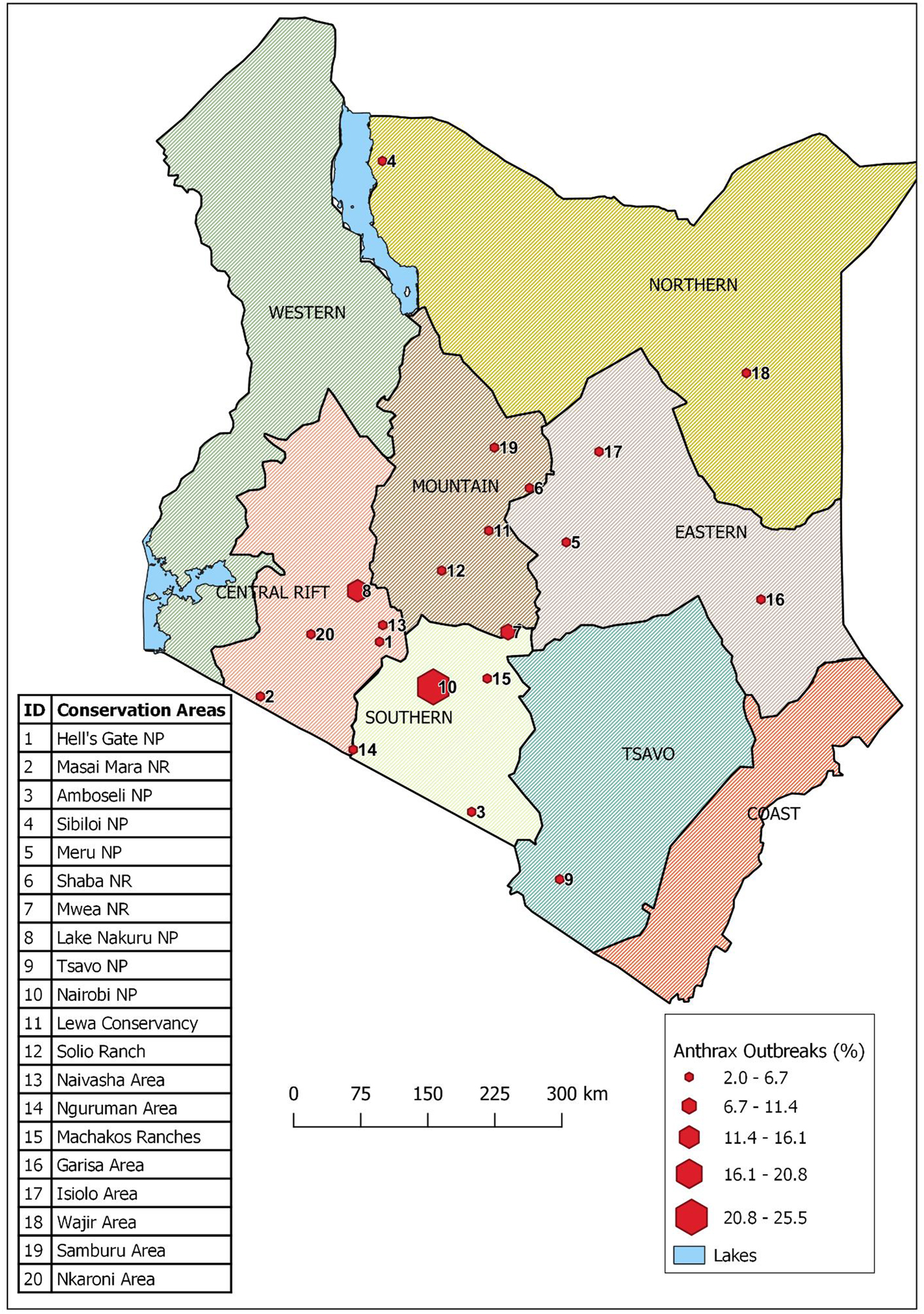
Fig. 1. Map of Kenya showing the eight administrative wildlife conservation regions (shaded in different colours) and the conservation areas (red hexagons) where the 51 anthrax outbreaks were reported during 1999–2017. NP refers to national park, NR refers to national reserve.
Results
Distribution of anthrax outbreaks and mortalities by conservation areas
Between 1999 and 2017, 51 anthrax outbreaks were recorded in 20 of 60 wildlife conservation areas spread across seven of the eight wildlife conservation regions of the country, with the exception of Western region (Table 1, Fig. 1). Of these, 19 outbreaks (37%) were reported in Nairobi and Lake Nakuru conservation areas, whereas the other 32 outbreaks (63%) occurred across 18 conservation areas. A total of 1014 wild animal deaths associated with the 51 anthrax outbreaks were recorded, with 81% (N = 816) of these deaths occurring in the Lake Nakuru conservation area during six separate outbreaks.
Table 1. Distribution of anthrax outbreaks and mortalities by conservation area, county and agro-ecological zone

When we defined the dominant AEZ for each conservation area where an anthrax outbreak occurred, we found that the 51 outbreaks were spread across six of the seven AEZs, with the exception of the agro-alpine zone that did not have any reported outbreaks (Table 1).
Distribution of outbreaks by species
The anthrax outbreaks affected 24 different wildlife species. Of the 51 outbreak records, buffaloes (23.5%), black rhinos (21.6%) and elephants (17.6%) were the most frequently affected species (Table 2). Except for few lions and foxes (two species) that were involved in two outbreaks, the other animal species involved in outbreaks were 22 herbivore species, including 12 (54.5%) with grazers, five (22.7%) with browsers and five (22.7%) with mixed grazers and browsers (Table 2) implying grazer and mixed grazer/browser herbivores were threefold more frequently affected than browser (77% vs. 23%).
Table 2. Frequency of wildlife species involvement in the anthrax outbreaks and the species food niche

Temporal and seasonal distribution of outbreaks
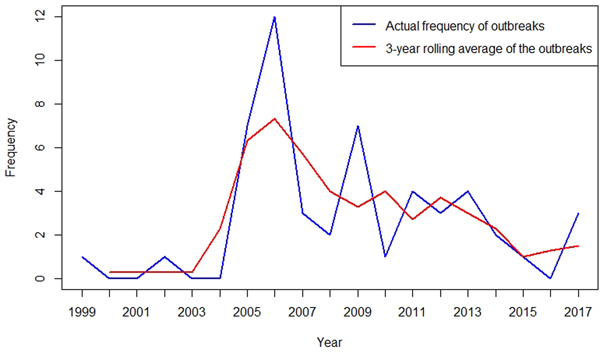
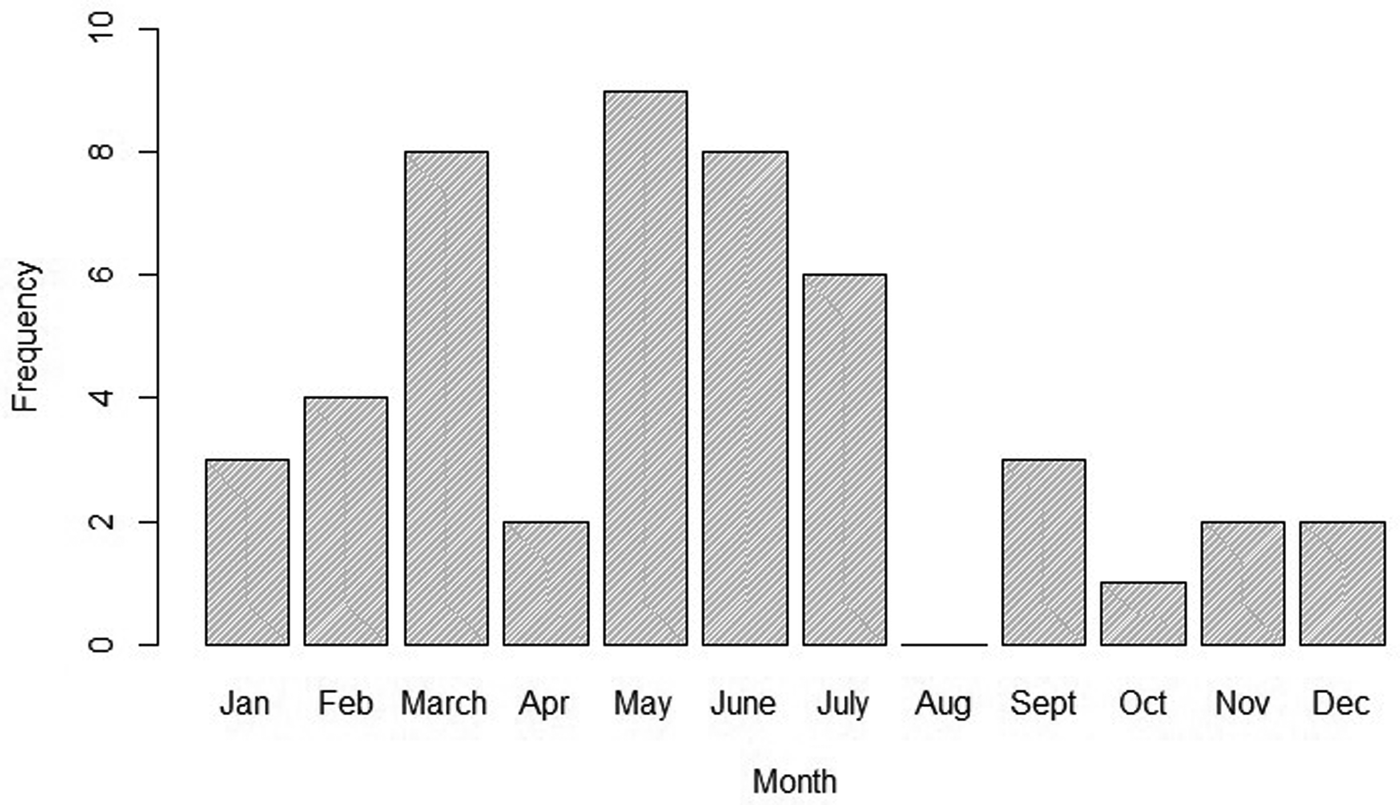
To better describe the temporal trend of anthrax outbreaks, a 3-yearly rolling average of the outbreaks from 1999 to 2017 was considered (Fig. 2). The temporal distribution of outbreaks between 1999 and 2017 shows an increase in the frequency of occurrence between 2005 and 2017 (Fig. 2). When classified by month of occurrence, the data show that of 48 outbreaks with information, 32 (67%) occurred during the dry months, including 15 (31%) in the dry and hot season between January to March, and 17 (36%) in the dry and cold months of June to September (Fig. 3). In contrast, 16 (33%) occurred during the rainy seasons including 11 (23%) during the long rain of April and May and five (10%) during the short rains of October to December (Fig. 3).
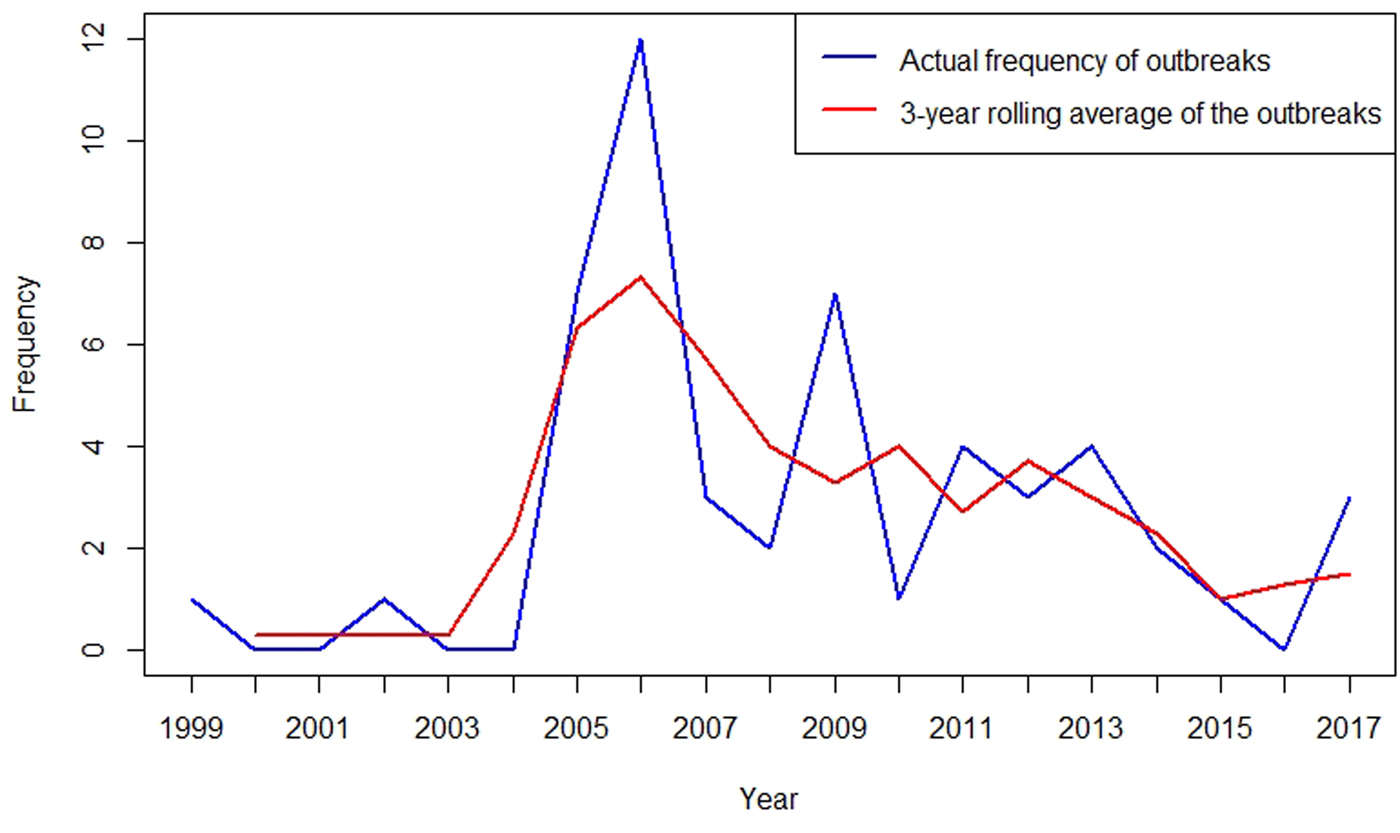
Fig. 2. Three-yearly rolling average of anthrax outbreaks in Kenyan wildlife, 1999–2017.

Fig. 3. Monthly distribution of anthrax outbreaks in Kenyan wildlife between 1999 and 2017.
Discussion
The findings of this study demonstrated the risk posed by B. anthracis to the wildlife population, illustrated by an outbreak at Lake Nakuru national park in 2015 that resulted in the death of 10.5% of all wildlife herbivores in the park, including 17% of the buffalos and 16% endangered black rhinos and white rhinos. Even though outbreaks were reported in <50% of all the wildlife conservation areas in Kenya, we suspected that the outbreaks were more widespread but unreported because of the inherent weaknesses of detecting wildlife deaths in the presence of scavengers.
Our data show that anthrax outbreaks were reported in both dry and wet seasons, however two-thirds of the outbreaks occurred during the 7 months of the dry seasons (January, February, March, June, July, August and September) in Kenya, perhaps suggesting that reduced moisture content is more important than temperature in the occurrence of outbreaks. This finding is consistent with other studies and could be attributed, in a limited way, to increased soil ingestion and spore inhalation during animal feeding when vegetation is depleted [Reference Turner16–Reference Mwakapenje21]. It is likely that animals also exhibit increased geophagia (consumption of soil) during the dry seasons, particularly in areas with mineral deficiencies [Reference Ayotte22]. Additionally, there is a social mixing of animals, both wildlife and domestic animals that invade national conservation areas during the dry seasons in search of pasture and water. The dry season is also linked with stress in animals associated with nutritional deficiencies, heat and parasitism resulting in lowered host immunity [Reference Hugh-Jones and de Vos23, Reference Bengis, Miller and Fowler24]. During the dry seasons, vegetation becomes increasingly dry and abrasive and may increase the chances of broken mucosa in the mouth facilitating entry of ingested B. anthracis spores into the blood circulation [Reference Starr25]. Receding spore-contaminated water following flooding may also contaminate pastures thus establishing new foci of infection to grazer herbivores. Most wildlife conservation areas in Kenya are located in arid and semi-arid lands, similar to the situation in other countries in sub-Saharan Africa including Namibia, South Africa, Zimbabwe and Tanzania [Reference Clegg7, Reference Palmer, Peel and Kerley26–Reference Lembo28]. However, it is interesting to note that we did not find records of anthrax outbreaks in wildlife conservancies in agro-alpine regions of Kenya including the Aberdare and Mount Kenya national parks. This observation is consistent with our findings that more outbreaks occur during the dry season, and it may be explained by the similar associated risk factors [Reference Muturi12].
The other finding in our study was that >90% of the wildlife species affected by anthrax outbreaks were herbivore, primarily because of their well-documented high susceptibility to B. anthracis infection when compared to carnivores and primates [Reference De Vos, Turnbull, Coetzer and Tustin17, Reference Hugh-Jones and de Vos23, Reference Cormack-Gates, Elkin, Dragon, Williams and Barker29]. Among herbivores, we found that grazer and mixed grazer/browser herbivores were threefold more frequently affected than browser (77% vs. 23%). This finding is in contrast with observations from studies in southern Africa, including in Malilangwe wildlife reserve in Zimbabwe where browsers accounted for >75% of herbivore mortalities, >3-fold higher than that observed in grazers and mixed grazer/browser [Reference Clegg7, Reference Hassim30]. The different levels of susceptibility between grazers and browsers are attributed to feeding behaviour; grazers feed closer to the soil where B. anthracis spores are maintained thus increasing the risk of ingesting or inhaling the infective spores. However, the higher anthrax occurrence among browsers in southern Africa is attributed to necrophagic flies that transfer the spores from carcasses to high vegetation leaves and twigs that browsers feed on [Reference Clegg7, Reference Braack and de Vos19, Reference Shiferaw31, Reference Blackburn32].
Apart from acquiring B. anthracis from vegetation and soil during feeding, it is suspected that the behaviour of wildlife associated with caring for the sick and dying may lead to exposure [Reference McComb, Baker and Moss33, Reference Goodall34]. Certain herbivore species, including buffaloes, tend to sniff and lick carcasses of their own species, increasing the chance of ingesting or inhaling anthrax spores. This mechanism may have played a major role during an anthrax outbreak in Lake Nakuru National Park in 2015 where >750 buffalos, constituting 17% of the species population at that national park died of anthrax in one outbreak [Reference Muturi12]. Some studies have also suggested that certain B. anthracis strains may have a higher predilection and/or virulence towards certain host species [Reference Hugh-Jones and de Vos23]. This is noteworthy considering that few genotypic studies of B. anthracis have been carried out in sub-Saharan Africa [Reference Smith35].
This study had some limitations. Isolated anthrax deaths in wildlife may go undetected because of quick action of scavengers to clear the carcass, and because the outbreak occurred in remote inaccessible locations. Molecular methods were not used to confirm the presence of B. anthracis; therefore, it is possible that microscopy may have misidentified other Bacillus spp (e.g. B. cereus biovar anthracis) as B. anthracis. However, no other Bacillus spp has been associated with widespread die-offs in mammals nor reported as a contaminant in blood in characteristically large numbers in anthrax deaths in Kenya to invalidate the conclusions.
Buffaloes (23.5%), black rhinos (21.6%) and elephants (17.6%) were the most frequently affected species. Our findings demonstrate the extensive geographic distribution of wildlife anthrax in the country, making it one of the important infectious diseases that threaten wildlife conservation.
In conclusion, our findings reveal the widespread geographic distribution of wildlife anthrax in Kenya, mostly affecting the large mammals important in conservation efforts. These findings underscore the need for greater empirical attention consisting of better wildlife disease surveillance and research, perhaps seasonally focused, for early detection of anthrax and control programmes.
Acknowledgements
We thank the Kenya Wildlife Service staff that assisted in availing annual reports, specific case reports, surveillance reports and post-mortem reports. We are also grateful to Grace Chege who abstracted data into the study database. Funding for the project was provided by the Defense Threat Reduction Agency (DTRA) Grant/Award #: HDTRA11710043.
Conflict of interest
None.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.