INTRODUCTION
Staphylococcus aureus is carried on the skin or mucosa of healthy individuals without causing harm, but it can cause severe infections and bacteraemia with high mortality [Reference Cosgrove1]. Infection with methicillin-resistant S. aureus (MRSA) is associated with increased morbidity, mortality and length of hospitalization compared to methicillin-susceptible S. aureus (MSSA) [Reference Cosgrove2, Reference Delaney3] and patients colonized with MRSA are at a greater risk of MRSA infection compared with non-colonized patients [Reference Davis4].
In 2008, about 16% of the UK population was aged >65 years [5], with the fastest population increase in England associated with those aged >85 years, with numbers doubling to 1·3 million [6]. By 2033 it is predicted that the number of people aged >85 years in England will double again to 3·2 million, accounting for 5% of the population. Notably, the proportion of older people in care homes increases rapidly with age, with 12% of men and 23% of women aged ⩾85 years in residential care in England. Being a closed community, care-home residents may be at a higher risk of S. aureus carriage and infections and are a source of potential transmission of MRSA both inside and outside the home [Reference Drinka7–Reference Jernigan9].
Only four previous studies have investigated the prevalence and risk factors associated with MRSA in UK care homes – estimating the prevalence of MRSA to range from 0·14% to 22% [Reference Cox and Bowie10–Reference Barr13]. Univariable analysis from these studies shows that MRSA carriage is associated with: male gender, age >80 years, previous antibiotic treatment, skin condition, invasive devices, vascular disease, ⩾6 beds per registered nurse, hospital admission and length of stay [Reference Cox and Bowie10–Reference O'Sullivan and Keane14]. However, after multivariable analysis, only male gender, age, invasive devices, hospital stay and number of beds per registered nurse remained significant.
The burden of dementia in care homes is high. Current estimates for the prevalence of dementia in individuals aged ⩾65 years living in Elderly Mentally Infirm (EMI) homes are 79·9%, nursing homes 66·9% and residential care homes 52·2% [15]. People with dementia are at risk of the same illnesses and risk of hospitalization as those without dementia. It is clear that residents with dementia have many characteristics which make infection control difficult, such as non-compliance with hand-washing, behavioural traits, frequent physical contact with care staff or other people. Yet residents with dementia are usually excluded from studies investigating MRSA in care homes because of the difficulties associated with enrolling vulnerable adults under the 2005 Mental Capacity Act for England and Wales [Reference Fraise11, Reference Barr13]. A large questionnaire study undertaken in Ireland 10 years ago, found that dementia was predictive of MRSA carriage in nursing homes, although dementia did not remain significant during multivariate analysis [Reference O'Sullivan and Keane14]. More recently, a study in 2006 demonstrated a statistically significant association between MRSA colonization and mortality of care-home residents with impaired cognitive function [Reference Suetens16].
It is important to know whether dementia is an independent risk factor for the carriage of S. aureus, both for the management of residents and for the validity of future studies investigating healthcare-associated infections. In this point-prevalence study we aimed to ascertain whether residents with dementia in nursing homes and residential care homes in Gloucestershire and Greater Bristol (UK) have an increased prevalence of MSSA and MRSA nasal carriage compared to residents without dementia. We also aimed to investigate other care-home and resident risk factors associated with the carriage of these organisms.
METHOD
Study population
The study was undertaken in Greater Bristol and Gloucestershire areas (mid-2007 population estimates 416 400 and 582 600, respectively) [17]; with care homes located in the inner city, urban and rural settings. Bristol has a higher 2007 Index of Multiple Deprivation score at 27·76 (ranked 49/152 where a ranking of 1 is the most deprived) compared with Gloucestershire, 14·65 (ranked 124/152) [18].
Homes providing old age and/or dementia services were identified in Greater Bristol and Gloucestershire via the Quality Care Commission (previously Commission for Social Care Inspection). Following stratification by the type of care provided, homes with at least 20 residents were randomly selected and invited to participate by telephone and letter from January 2008 until the end of the study in August 2009. All residents at participating homes were eligible for inclusion.
Sample size
A total of 750 residents were estimated, based on previously reported MRSA prevalence rates of 20% [Reference McNulty19]. This gave the power to detect an increase of MRSA prevalence of 15% in patients with dementia (expected prevalence 30%) [Reference McNulty19], 15% in patients hospitalized in the previous year (expected prevalence 26%) [Reference Barr13], 20% in urinary catheterized patients (expected prevalence 8%) [Reference McNulty19] and 15% in male patients (expected prevalence 20%) [Reference Barr13].
Screening residents for dementia
Presence of dementia was determined with the clock-drawing test and was administered in accordance with previously published methods [Reference Shulman20]. The test is easy to administer, appears to be well tolerated by patients and is not influenced by language, culture, ethnicity or education [Reference Shulman21]. The test requires a mix of cognitive skills, including visuo-spatial abilities and executive control functions [Reference Shulman20]. We graded the test results using the scoring system of Shulman [Reference Shulman20], which has been shown to have good sensitivity and specificity compared with other validated methods of cognitive function, especially the Mini-Mental State Examination [Reference Brodaty and Moore22–Reference van der Burg24]. Furthermore, inter-rater and non-expert agreement has been shown to be good [Reference Brodaty and Moore22–Reference van der Burg24]. For residents who were unable to participate in the clock-drawing test, due to poor eyesight or physical disabilities, we used the 10-question Abbreviated Mental Test (AMT) score. A score of >6 in the AMT reliably rules out dementia [Reference Antonelli Incalzi25].
Ethics and consent
Approval was gained from the local research ethics committee (LREC no: 06/Q2005/99). A senior member of staff gave written informed consent for the care home to participate.
At the first visit to a participating home, researchers approached only residents who were considered by care-home staff to have the capacity to consent. These residents were asked to complete a dementia assessment exercise and for informed oral consent to a nasal swab. Individuals who did not have the capacity to consent or those initially considered by home staff to have the capacity to consent but later failed the clock-drawing exercise were not excluded from the study. Residents who were considered too ill or frail to participate were excluded. Assent was sought from a resident's nominated or personal consultee in accordance with the Mental Capacity Act 2005. Letters requesting assent were sent to the appropriate person and non-respondents were followed up after 2 weeks with an additional letter. Any residents without an appropriate consultee acting on their behalf or without assent were excluded from the study.
Residents for whom consent or assent had been obtained, but were later found unwilling to cooperate when approached by researchers wishing to take a nasal swab, were given a second opportunity on a different day to be involved with the study. This was considered important due to the nature of dementia and the daily variation in understanding. Refusal on both occasions was considered a refusal of consent and therefore the resident was excluded from the study.
Microbiological survey
On a separate care-home visit, swabs were taken from the anterior nares of residents for whom consent or assent had been given. A researcher or carer asked residents to blow their nose, then the inside rim of the nostril was carefully wiped for about 5 s with a sterile cotton-tipped swab, which was then inserted in Amies charcoal transport medium (TCS plc, UK) and sent to Gloucestershire Royal Hospital Microbiology Department for processing. Each swab was cultured within 24 h on Columbia blood agar (Oxoid, UK) and MRSA selective chromogenic agar (bioMérieux, UK) using standard methods as previously described [Reference Brown26, 27]. Isolates were identified as S. aureus by standard diagnostic tests and methicillin resistance was confirmed by resistance to cefoxitin (10 μg disk; Oxoid) [27, 28].
Data collection
Care-home staff completed a paper questionnaire about residents and care-home risk factors that might be associated with MRSA or MSSA carriage. Information was collected regarding resident's age, gender, length of time at the home, level of dependency, level of dementia from previous assessment, ability to move around the home unassisted, hospital admission, surgical procedure in last 6 months, history of MRSA carriage/infection, chronic wound or skin lesions, invasive devices, shared room, use of communal areas, hand to nasal contact, ability to independently wash hands and frequent physical contact with staff or other residents. Care-home risk factors included home type, service provider, number of residents, categories of residents, details of staffing levels, facilities at the home and written infection control policies.
Data analysis
Associations between nasal carriage of either MSSA or MRSA and patient, healthcare or care home-related factors were assessed using χ2 tests of association and Fisher's exact test. Estimates of relative risk ratios (RRR) were obtained using multinomial (polytomous) logistic regression analysis, with the three categories of MRSA, MSSA or no S. aureus nasal carriage as the outcome variable. Initially, the heterogeneity in carriage prevalence of MRSA, MSSA and all S. aureus between the care homes was assessed by the use of funnel plots. While there was no strong evidence of heterogeneity the potential lack of independence of carriage of residents within a care home was incorporated into the regression model by estimating standard errors of the estimated odds ratios that allow for the ‘clustering’ of residents. In the regression models, Wald tests were used to assess the associations. All analyses were performed using Stata version 10 (StataCorp., USA).
RESULTS
Care homes and residents
In total 51 homes were recruited; 14 residential care homes (7 Gloucestershire, 7 Greater Bristol) and 37 nursing homes (21 Gloucestershire, 16 Greater Bristol). These homes served 1881 residents, of which 361 were too frail to approach. Of the remaining 1520 residents, 772 (51%) residents were excluded; 616 (40%) did not provide consent or assent, 151 (10%) had no nasal swab taken and a remaining five consenting residents were unable to complete a dementia screen (i.e. due to incapacity, inability to communicate or lack of interest). Thus we obtained nasal swabs from 748 (49%) consented or assented residents. The median number of residents recruited from a care home was 13 (range 5–34). The mean age of residents excluded and included was similar (excluded 85·9 years, s.d.=8·7; swabbed 85·7 years, s.d.=8·3; P=0·7), but those excluded were significantly more likely to be female [excluded 601 (78%); swabbed 548 (73%); P=0·02].
Prevalence of S. aureus
Of the 748 recruited residents, 179 (23·9%, 95% CI 20·9–27·0) were culture positive for S. aureus. Of these 121 (16·2%, 95% CI 13·5–18·8) were MSSA and 59 (7·8%, 95% CI 5·8–9·7) were MRSA. There was no strong evidence of a relationship between the carriage of MRSA and MSSA in the 51 care homes (Spearman's correlation 0·1, P=0·23), in fact only one resident carried two strains of S. aureus; two MRSA strains, with distinct morphologies and antibiogram patterns. In nine of the homes (18%), no residents had nasal carriage of MSSA or MRSA.
The median prevalence of MRSA carriage was 5·9% (lower quartile 0·0%, upper quartile 11·8%), with some evidence of a difference in MRSA carriage between care homes (P=0·05). The prevalence in one home did exceed the upper 95% confidence limits, but this is an expected observation in a study of this size.
The median prevalence of MSSA was 16·7% (lower quartile 4·8%, upper quartile 24·0%). There was some evidence of a difference in MSSA carriage prevalence between care homes (P=0·005). For none of the homes did the estimated prevalence exceed the upper 95% confidence limit; however, for 12 homes with between 6 and 16 recruited residents there was no carriage.
Dementia screening
Dementia was present in 499 (66·7%, 95% CI 63·3–70·1) of the 748 residents that had a nasal swab. The AMT was undertaken by 272 residents; 126 (46%) attained a score of ⩾7 (which excluded dementia) and 146 (54%) residents were considered unable to provide informed consent. The clock-drawing exercise was undertaken by 353 residents; 147 (42%) residents failed to accurately complete the clock-drawing exercise, indicating some level of cognitive impairment and therefore an inability to provide informed consent. A further 290 residents, initially identified by care-home staff as capable of providing informed consent, were considered to have dementia as they were unable to provide consent or attempt an exercise.
Risk factors for nasal carriage of MSSA and MRSA
There was no evidence to suggest that nasal MSSA or MRSA carriage differed in residents with or without dementia (Table 1). Of the 499 residents screened or otherwise considered to have some level of dementia, 84 (16·8%) carried MSSA, compared to 37 (14·9%) of the 249 non-dementia residents (P=0·5). While 39 (7·8%) of the residents considered to have dementia carried MRSA, compared to 19 (7·6%) of the non-dementia residents (P=0·9). In those residents with dementia there was no evidence of a difference in nasal MRSA carriage in those given the AMT (4/77 5·2%), clock-drawing test (9/131 6·9%) or who assented (27/288 9·4%) (P=0·5).
Table 1. Association between resident-related factors and nasal MSSA and MRSA carriage
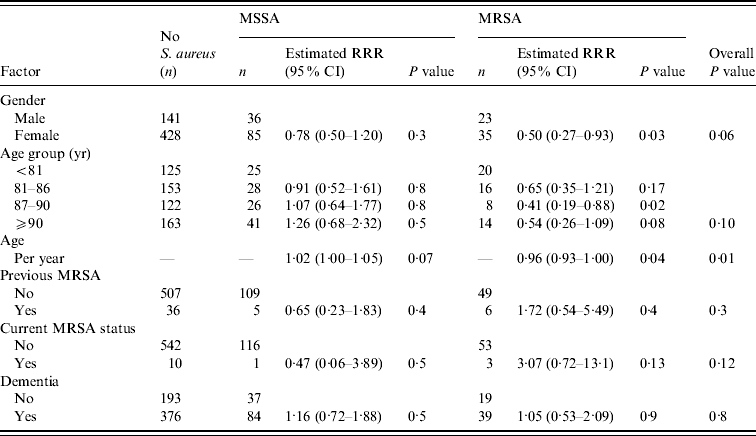
RRR, Relative risk ratio; CI, confidence interval.
The results from the single variable analysis for all risk factors are presented in Tables 1–3. Many of the risk factors investigated were not significantly associated with carriage of MSSA or MRSA. There were nine factors from the single variable analysis that exhibited some association with nasal S. aureus carriage: gender, age, urinary catheterization, admission to hospital in the past 6 months, level of dependency, ability to move around the home unassisted, independently wash hands, number of residents in the home, and home location (Gloucestershire or Greater Bristol). These factors were entered into a multivariable polytomous logistic regression analysis to assess whether any of these associations could be explained on the basis of confounding. The association for each of these was assessed after allowing for the other variables. If any variable did not exhibit an association, its association with the other variables in the regression model was assessed in order to determine which, if any, could provide an explanation.
Table 2. Association between healthcare-related factors and nasal MSSA and MRSA carriage
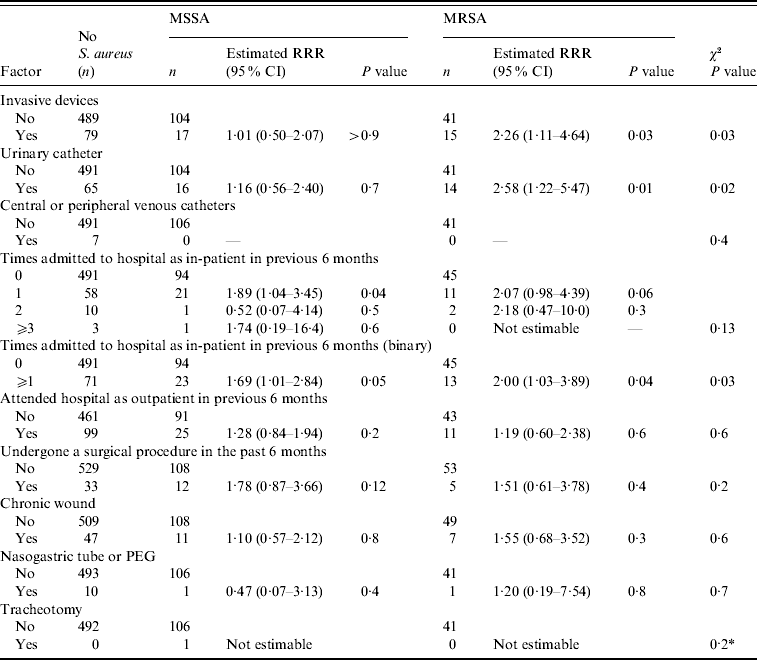
RRR, Relative risk ratio; CI, confidence interval; PEG, percutaneous endoscopic gastrostomy.
* Fisher's exact test.
Table 3. Association between care-home-related factors and nasal MSSA and MRSA carriage
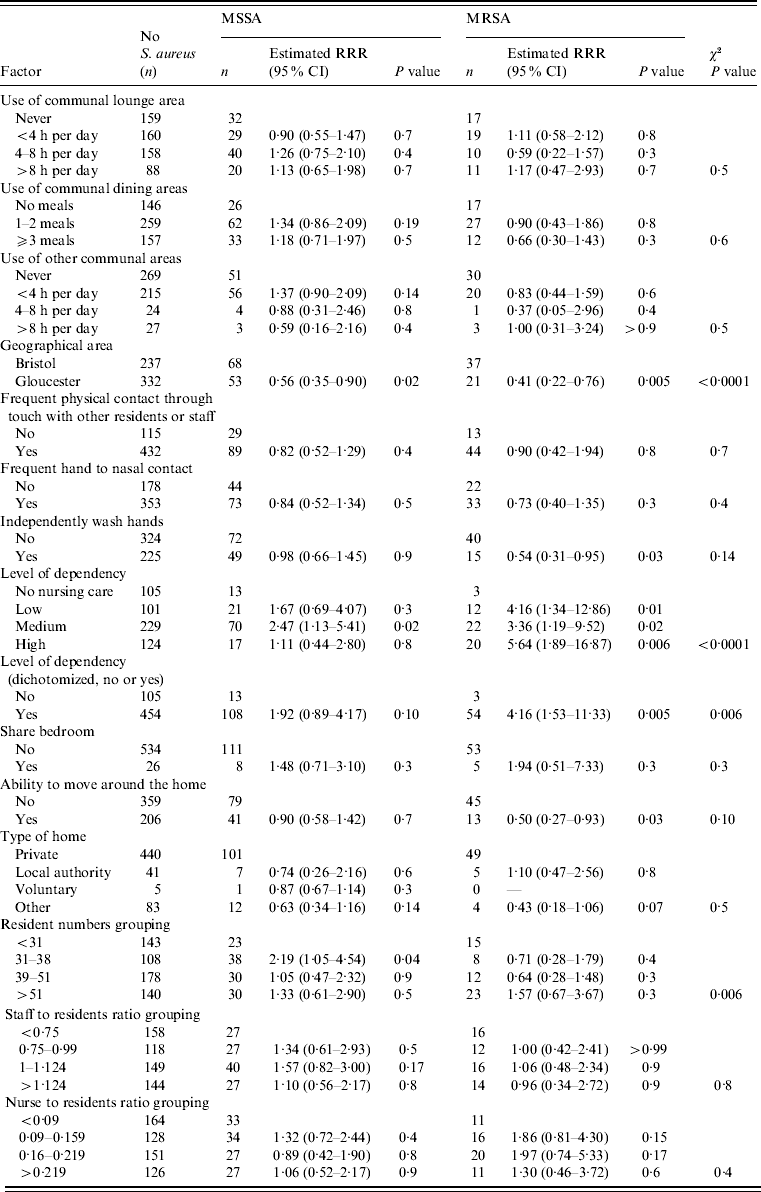
RRR, Relative risk ratio; CI, confidence interval.
When assessed in a model with all nine variables, there was no longer an association between independently washing hands and MSSA or MRSA carriage (P=0·6). This was due to the strong association between independently washing hands and the ability of the resident to move around the home. Of the 434 residents that could not independently wash their hands, only 69 (15·9%) residents had the ability to move around the home. Of the 287 residents that could independently wash their hands, 173 (60·3%) were able to move around the home. Therefore the previously observed association between nasal MRSA carriage and independently washing hands could be explained by the confounding effect of the resident's ability to move around the home, which appeared to be associated with both independent hand washing and MRSA carriage.
After removing independently hand washing from the regression model, the association between nasal MRSA carriage and urinary catheterization was also not significant (P=0·5). On removal of urinary catheterization from the regression model, there was no strong evidence of an association between level of dependency and MSSA or MRSA carriage (P=0·17). Further, after the removal of independently hand washing, urinary catheterization and level of dependency from the regression model, there was no strong evidence of an association between gender and the carriage of MSSA or MRSA (P=0·14).
The remaining five variables (age, region, ability to move around the home unassisted, hospital admission, and number of residents per home) all had some evidence of an association with nasal MSSA or MRSA carriage (Table 4). For age there was an unusual association, with MSSA carriage increasing as age increased (P=0·03), while MRSA carriage was found to decrease with increasing age (P=0·05). Those residents able to move around the home unassisted were at a lower risk of MRSA carriage (RRR 0·53, 95% CI 0·30–0·98, P=0·04); however, this variable did not appear to be associated with MSSA carriage (RRR 0·91, 95% CI 0·56–1·47, P=0·7).
Table 4. Association between variables and nasal MRSA carriage in the multivariable model
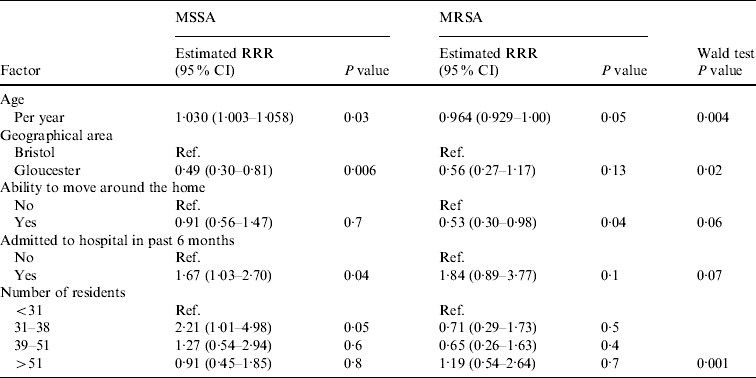
RRR, Relative risk ratio; CI, confidence interval.
For both MRSA and MSSA there was some evidence to suggest that those residents admitted to hospital within the past 6 months were more likely to have nasal carriage (MRSA: RRR 1·84, 95% CI 0·89–3·77, P=0·10; MSSA: RRR 1·67, 95% CI 1·03–2·70, P=0·04).
Nasal MSSA carriage was significantly lower in Gloucestershire care homes while MRSA carriage showed a similar reduction that was not significant (MSSA: RRR 0·49, 95% CI 0·30–0·81, P=0·006; MRSA: RRR 0·56, 95% CI 0·27–1·17, P=0·13) when compared to homes in Greater Bristol. There was a significant association with the number of residents per home for MSSA, with those homes with 31–38 resident exhibiting an increase in carriage compared to all other groups (P=0·006). While the number of residents was not associated with MRSA carriage (P=0·2).
DISCUSSION
We found that dementia was not a significant risk factor for the carriage of either MSSA (16·2%) or MRSA (7·8%) in care-home residents. However, residents able to move around the home unassisted were at a lower risk of MRSA carriage (P=0·04). Carriage of MSSA increased with increasing age (P=0·03), while MRSA carriage decreased. Hospitalization in the last 6 months increased the risk of both MSSA (P=0·04) and MRSA (P=0·10) carriage.
O'Sullivan & Keane in 2000 [Reference O'Sullivan and Keane14] investigated risk factors associated with MRSA colonization via a retrospective questionnaire, completed by 786 residents screened for MRSA, living in six Eastern Health Board long-term residential care facilities. They identified cognitive impairment as a risk factor predictive of higher MRSA carriage rate; however, it did not remain an independent risk factor significant on multivariable analysis. In our study, researchers approached and assessed the mental capacity of 815 residents using validated and robust dementia assessment tools [Reference Shulman20–Reference Antonelli Incalzi25]. From these in-depth assessments, we found that diminished cognitive impairment was not associated with a higher rate of MSSA or MRSA. Therefore excluding residents with dementia from future care-home studies investigating S. aureus carriage would not be detrimental.
A similar care-home study conducted in Leeds by Barr and colleagues found that age was not associated with MRSA colonization [Reference Barr13]. They assessed 715 residents from 39 care homes, with a median age of 85 years (range 61–103 years). The most significant age-related finding was associated with those residents aged ⩾90 years, with the crude odds ratio on single variable analysis of 1·45, suggesting that age may have been a causative risk factor; however, the 95% confidence interval crossed unity (0·85–2·48) and, therefore, a definite association could not be determined. In Gloucestershire, we found that residents aged <81 years were significantly more likely to carry MRSA, whilst MSSA carriage was significantly higher in residents aged >90 years.
Our study suggests that the risk of colonization varies with geographical area, with MSSA carriage lower in Gloucestershire homes compared to Greater Bristol homes and lower than in Leeds in 2005, which showed an MRSA prevalence of 22% [Reference Barr13]. Differences may also be due to hospital rates of MRSA. Although our study was not designed to determine this, mandatory data on bacteraemia rates does give an indication of MRSA rates in these areas; Leeds Teaching Hospital rate for 2004/2005 at 2·62 compared to North Bristol and University Hospitals Bristol in 2007/2008 with rates of 2·10 and 1·59, respectively. Clearly more work is needed to understand the variables associated with regional differences in carriage rates.
The ability of residents to move around the home unassisted was found to be protective for MRSA carriage, but not significant for MSSA colonization. This finding suggests that those residents reliant on staff assistance for care and movement around the home were at a higher risk of MRSA carriage. This was also indicated in the single variable analysis as level of dependency, inability to wash hands independently and presence of a urinary catheter were all associated with increased carriage of MRSA. This result suggests that residents are acquiring MRSA via cross-infection due to staff members and demonstrates the need for stricter hygiene standards in care homes.
Other studies have identified hospitalization as a risk factor for MRSA colonization [Reference Lucet8–Reference Namnyak12, Reference Coia29, Reference von Baum30]. Our study also found that residents recently admitted (⩽6 months) to hospital as in-patients were at a higher risk of both MRSA and MSSA carriage. This supports the widely held view that hospitals act as a reservoir for S. aureus, with residents returning to care homes after acquiring strains endemic in the hospitals. However, more work is required to investigate whether this is the case and the level of transmission via this route; this would necessitate detailed type identification of care-home isolates and their corresponding regional hospitals.
Previous work into the colonization of the anterior nares with S. aureus has shown that it is unusual for individuals to be colonized with more than one strain of S. aureus or both MSSA and MRSA, suggesting that colonization with MSSA is protective and prevents individuals becoming colonized with MRSA [Reference Dall'Antonia31]. This explains why no residents were identified in our study with co-colonization of both MSSA and MRSA. In fact the only resident with more than one S. aureus isolate was found to be carrying two different strains of MRSA.
The original sample size calculations made assumptions, based on published work, that 20% of residents would have nasal carriage of MRSA and 30% of residents would have dementia [Reference Barr13, Reference McNulty19]. The observed MRSA carriage was about 8%, while the prevalence of dementia was 67%. This has the effect of reducing the power of detecting a relative increase in carriage of 75% from 90% to 50%. However, the 95% confidence intervals around the estimated increase in carriage would exclude a doubling of S. aureus carriage in those with dementia.
To our knowledge, this is the only UK study that has assessed care-home residents for dementia; enrolling residents with cognitive impairments and assessing the burden of S. aureus carriage within this cohort. We recruited a wide range of nursing and residential care homes, including a selection of private, voluntary and local authority homes. All care homes were approached in a random order and those homes unwilling to participate were similar to those enrolled. It is, therefore, likely that the 51 care homes recruited were an unbiased representation of the types of homes throughout Gloucestershire and Greater Bristol and representative of other homes in the UK.
In line with the Mental Capacity Act 2005, a large number of care-home residents were not recruited due to the rigorousness of our assent procedure [32]. Nonetheless, excluded residents were similar in terms of age when compared to those residents who took part in the study. A recent study found that nasal swabs alone detected only 75% of all MRSA-colonized subjects [Reference Peters, Redding and Allardice33]. Due to the frailty and severe dementia of many of the residents enrolled in our study, researchers were only able to obtain a nasal swab for the investigation of staphylococcal carriage. Thus, our results may be an underestimate of the true prevalence of S. aureus in the care-home setting, but there was no practical alternative for investigating carriage in this cohort.
We performed direct culture of all nasal swabs onto non-selective and chromogenic agar. Although some previous research indicates that the enrichment broth method is more sensitive for isolation of MRSA [Reference Brown26], the introduction of new chromogenic agar media over the last few years has brought this finding into question. Notably the UK National Standard Methods recommend routine screening by direct plating on chromogenic medium, commenting that the advantage of enrichment over direct plating has yet to be confirmed with chromogenic medium [27]. Additionally, recent research has shown that incubation of chromogenic agars for 48 h significantly increases the sensitivity of these media [Reference Nahimana, Francioli and Blanc34, Reference Nonhoff35].
Clearly the control of MSSA and MRSA in care homes requires more focus on infection control interventions, such as the National Patient Safety Agency ‘clean your hands’ campaign [36] or the recent Health Protection Agency DVD on the ‘Introduction to infection control in care homes’ [37]. In addition, the confirmed relationship of MRSA with recent hospitalization stresses the importance of controlling MRSA in the hospital setting, as this should reduce colonization and morbidity in care homes.
ACKNOWLEDGEMENTS
This study was centrally funded by the Health Protection Agency. Special thanks to staff, residents and relatives at participating care homes for their involvement and enthusiasm. The authors acknowledge the invaluable assistance of Kim Turner, Reagan Blyth and Julia Hande at the PCU, HPA Gloucester, for recruiting and attending care homes, screening, recruiting and swabbing residents and administrative support; Diane Webb, Anne Maher and Wendy Nedoma from the Vaccine Evaluation Unit, HPA Gloucester, for screening, enrolling and swabbing residents; Gloucester RDSU for their invaluable advice and support; David Tompkins, Yorkshire & the Humber Regional Microbiology, HPA, for guidance during study development; Mel Jones, HPA SW, for advice during study development; Cheryl Haswell, Health Protection Unit, for helping with care-home recruitment at study onset; Jill Whiting, PCU, HPA Gloucester, for general and administrative support; Anjeet Jhutty, Gloucestershire University, for administrative support and enrolling residents; the staff of Microbiology laboratories at Frenchay Hospital and Gloucestershire Royal Hospital, NHS Trust, for their support and laboratory space.
DECLARATION OF INTEREST
None.






