INTRODUCTION
Salmonella is a major cause of foodborne disease in both the industrialized and the developing world [1–Reference Mølbak, Olsen, Wegener, Riemann and Cliver3]. Although Salmonella is a frequent cause of foodborne outbreaks, the majority of the reported cases are sporadic. Infections are frequently associated with foodborne transmission, but other routes of exposure, such as direct contact with live animals and person-to-person transmission, have also been identified [Reference Baker4–Reference Engberg7]. Identifying the most important sources of human foodborne disease is essential for prioritizing food-safety interventions and setting public health goals [Reference Pires8].
Several types of studies have been performed to identify possible sources of apparently sporadic human infections. Case-control studies are the most commonly used analytical epidemiological approach. Typically, selected patients (cases) and a corresponding group of asymptomatic and therefore assumed uninfected individuals (controls) are interviewed, and the relative role of exposures is estimated by comparing the frequency of exposures among cases and controls. When infections are associated with an exposure, the proportion of cases attributed to the exposure can be calculated and this measure is defined epidemiologically as the ‘population attributable fraction’ (PAF) [Reference Clayton and Hills9]. PAFs can be used to partition the human disease burden to specific sources [Reference Stafford10]. Alternatively, the relative importance of risk factors can provide an indication of which sources or routes of exposure are responsible for a higher burden of disease. Case-control studies are a valuable tool to identify potential risk factors for human infections, including sources and predisposing, behavioural or seasonal factors [Reference Engberg11]. In addition to individual case-control studies, a systematic review (SR) of published case-control studies of sporadic infections of a given foodborne disease can provide a comprehensive summary of the estimated measures of association and PAFs for each exposure, and this can be combined to estimate the overall burden of illness attributed to each exposure.
SRs consist of a formal process for literature review focused on a specific research question, and include the identification of relevant literature, quality assessment of relevant studies, summarization or statistical analysis of data, and conclusions [Reference Khan12, Reference Sargeant13]. The intent of SRs is to apply review methods that minimize systematic and random errors, and thus minimize the introduction of bias and provide reliable basis for the decision-making process. Meta-analysis consists of an analysis of the summarized statistics of the studies provided by the SR.
This SR and meta-analysis aimed at comparing the relative importance of risk factors for cases of Salmonella, thus providing information for source attribution of human salmonellosis and delineation of interventions to reduce the burden of disease.
MATERIALS AND METHODS
Literature search
A literature search was conducted in February 2008, and was limited to the languages English, German, Portuguese, Spanish, and Danish. Relevant studies were identified using a combination of key words in the databases Medline, Science Direct, Agricola, CAB International, Biosis, FSTA, ISI Web of Science, and Web of Knowledge. In addition to published peer-reviewed studies, relevant studies published as conference proceedings and in scientific reports were also searched. A combined search was performed, looking for case-control studies of Salmonella spp. and Campylobacter spp. sporadic infections.
The search was conducted using a combination of (1) general terms, related to case-control studies and risk factors, and (2) Campylobacter and Salmonella terms. Citations were collected, de-duplicated and managed in web-based software (SRS 4.0, TrialStat! Corporation, Canada).
An additional traditional literature search, using the same search terms but without assistance of SR software, was performed in February 2010, and new references were added to the previously retrieved studies.
Relevance screening
All references were independently reviewed by two reviewers, and it was sufficient that one reviewer considered it relevant for the reference to pass to the quality assessment step of the SR. Relevance of studies was assessed on the basis of specific inclusion criteria: (1) focus on human disease; (2) focus on Campylobacter or Salmonella; (3) focus on sporadic disease; (4) reference describing a case-control study.
Quality assessment
Methodological soundness was assessed by two reviewers on the basis of the following study quality criteria: (1) statistical power above 80%, if information was available (if the power of the study was not mentioned, the reviewers were asked to evaluate the reference based on the other criteria); (2) case definition implying laboratory confirmation of the diagnosis; (3) random selection of controls; (4) comparability of cases and controls; (5) control for potential confounding factors (matching); (6) acceptable matching criteria for matched study designs (e.g. age and gender); (7) exposure window for cases and controls acceptable (maximum 10 days) and comparable; (8) response rates for cases and controls acceptable; (9) appropriate statistics; (10) the studies provided the odds ratio (OR) with the 95% confidence interval (CI) of the effect of each exposure based on conditional logistic regression; (11) acceptable study design (the reviewers assessed the overall quality assessment criteria and decided on the inclusion or exclusion of the reference).
The non-compliance with a single criterion was not sufficient to reject a study. Instead the reviewer was asked to do an overall assessment based on all criteria, and studies not fulfilling two or more criteria were excluded. If the two reviewers disagreed on the acceptance of a study, a third reviewer was consulted.
Data extraction
Data from studies that passed the previous steps were manually extracted by one of two reviewers using a standardized form. The data extracted included country and time period of the study, age stratification of the population, study design parameters (e.g. matched or unmatched study), and outcome of the study (ORs for specific risk factors together with the 95% CIs).
Data analysis
Two separate meta-analyses were performed to compare and combine information from different studies. The meta-analysis presented here focuses on sporadic salmonellosis. The Campylobacter study will be described in a forthcoming paper [Reference Domingues14]. All risk factors were stratified according to source-categorization schemes and location of exposures.
Source categorization
Exposures were categorized in six main groups: food, direct contact with live animals, environment, person-to-person, predisposition and travel. Additionally, food preparation risk factors were included for specific food routes with the purpose of distinguishing between the impact of exposure through consumption of foods and through handling of food items.
Risk factors from the main groups food (Fig. 1) and direct contact with live animals were sub-categorized in a hierarchical scheme of mutually exclusive categories. Environmental transmission routes included drinking water, exposure to recreational waters, and exposure to contaminated environments (e.g. playgrounds) or objects (e.g. bottles). In general, categorizations were based on (1) main reservoirs of the pathogen, (2) main routes of transmission from the reservoir to the susceptible population, and (3) important predisposing and behavioural factors for human exposure (e.g. occupational exposure to farm animals or daily contact to pets). The main groups person-to-person, predisposition and travel were not sub-categorized. Predisposing factors included previous intake of drugs (e.g. antimicrobials and anti-acids), or pre-existing chronic disease, and were analysed individually.
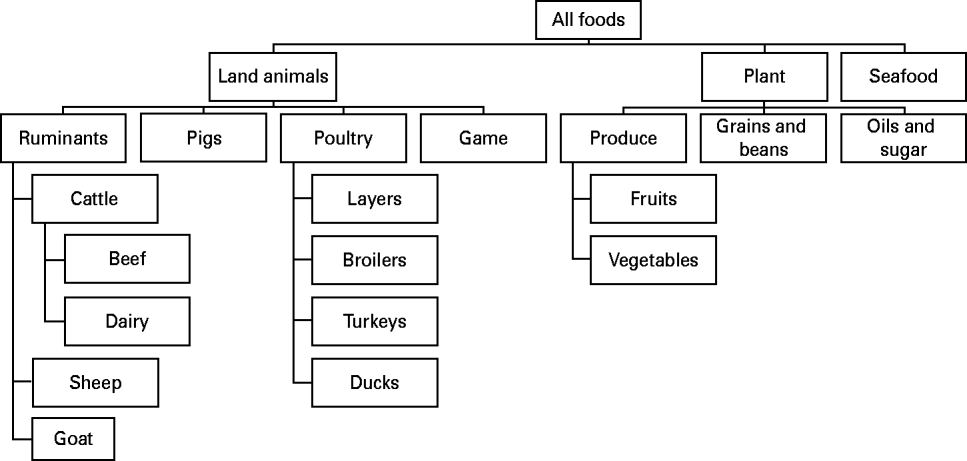
Fig. 1. Categorization of foods (based on [Reference Painter33]).
Location of exposures
Risk factors from the main group food were further classified as household or outside the household, according to the setting of the exposure. The location of exposure corresponds to where the food was consumed or exposure occurred (e.g. cafe/restaurant, institution, home).
Meta-analysis procedure
Outcome parameters
The ORs and 95% CIs per risk factor from each study were pooled in a meta-analysis using commercial software [15]. Some studies presented more than one risk factor that could be integrated in the same categorization stratum (e.g. ‘eating beef’ and ‘eating ground beef’). For these cases, a combined effect was calculated per study [15], so that a study with several risk factors in the same stratum did not have more influence on the total effect. When a study had more than one risk factor in the same main category (e.g. the food category chicken) but classified in a different location category (e.g. ‘eating chicken at home’ and ‘eating chicken outside home’), each factor was treated individually. Similarly, risk factors belonging to the same category but describing consumption of raw or undercooked foods were treated separately.
Studies are designed differently, conducted in different time periods and on different populations, which can create heterogeneous study populations [Reference Halasa16]. Moreover, if only a small number of studies for a risk factor is available, homogeneity between groups could be apparent, but this would be a consequence of low statistical power and not due to actual lack of differences. Therefore, a random-effects model was used to calculate the pooled ORs [Reference Borenstein17].
The meta-analysis was designed to assess the influence of the factors age of the study population, geographical region, and study period in the final outcome. Regional analyses were performed according to the United Nations regions (see http://www.un.org/depts/dhl/maplib/worldregions.htm). For each stratum, we calculated (1) a pooled OR and 95% CI per group (age, region, time period, serotype), and (2) a total pooled OR and 95% CI based on all groups [15]. The meta-analysis was performed only when at least four studies were available [Reference Halasa16, Reference Halasa18] for each stratum.
Publication bias
The publication bias was assessed using Duval & Tweedie's trim-and-fill method [Reference Duval and Tweedie19], Begg & Mazumdar's rank correlation test [Reference Begg and Mazumdar20], and Egger's regression test [Reference Egger21]. When significant publication bias and change in the estimated pooled ORs were detected, the number of studies necessary to reverse the overall pooled effect was calculated using Orwin's fail-safe N method [Reference Orwin22]. The influence of a single study was also examined using the one-study-removed method [Reference Dohoo, Martin and Stryhn23]. If significant publication bias existed, the pooled ORs were estimated after correcting for the bias, based on Duval & Tweedie's trim-and-fill method.
A significant publication bias was considered to exist when adjustment for the bias altered a previous conclusion or when the confidence limits of the unadjusted and the adjusted ORs did not overlap.
RESULTS
Systematic review
From 1295 identified references, 131 passed the relevance screening, 72 passed the quality assessment stage, and data was extracted from 71. Full text references could not be found for 13 references, which therefore did not pass to the data extraction phase. One reference was added after a posterior non-SR. Results of the SR process are summarized in Table 1.
Table 1. Systematic review statistics
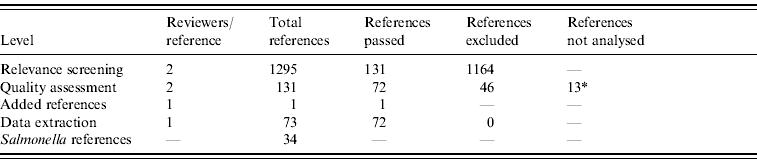
* Full text references could not be found, and a proper quality assessment was not possible.
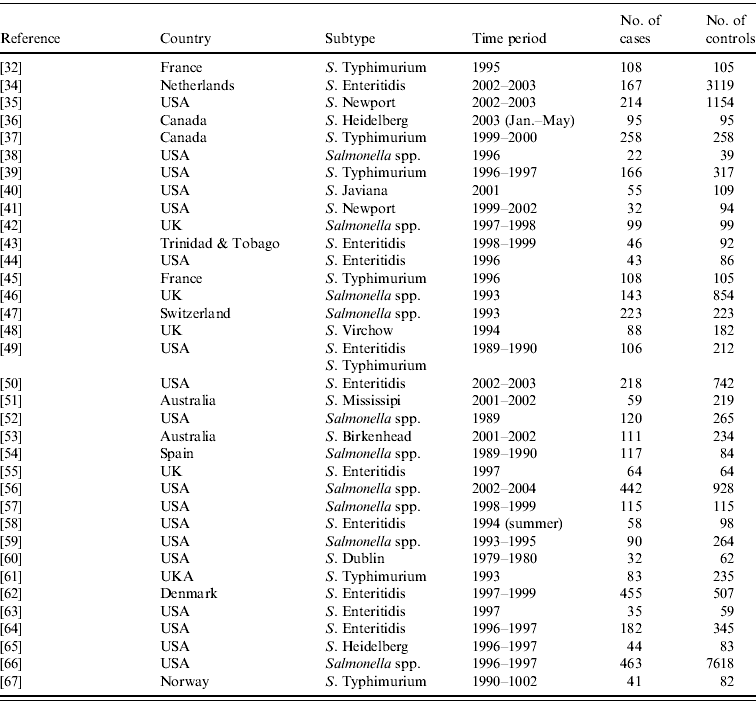
From the 72 references, 34 investigated risk factors of sporadic salmonellosis and 38 focused on sporadic campylobacteriosis [Reference Domingues14]. Salmonella case-control studies were conducted between 1989 and 2003 in 11 different countries. Two studies analysed exposures in children and five studies interviewed only individuals above 10 or 15 years of age. Some studies investigated risk factors for specific serotypes: 10 studies focused on S. Enteritidis, and few studies limited analyses to other serotypes. Overall, the number of cases and controls interviewed varied between 22 (small scale studies) and 7618 (community studies). All studies were published in English. The Appendix presents the complete list of Salmonella studies collected in the SR.
Meta-analysis of risk factors of human sporadic salmonellosis
Results show that international travel (OR 6·5, 95% CI 3·81–11), the previous intake of anti-acids (OR 2·9, 95% CI 1·5–5·7), pre-existing medical condition (OR 2·8, 95% CI 1·98–4), previous intake of antimicrobials (OR 2·23, 95% CI 1·65–3·00), eating raw eggs (OR 2·78, 95% CI 1·87–4·10), and eating in a restaurant (OR 2·74, 95% CI (1·74–4·32) were the most important risk factors for human salmonellosis in the overall study population (Table 2). Consumption of undercooked or raw eggs and chicken in a restaurant were the only food items identified as relevant for human disease in the analysis, and environmental routes (both drinking and recreational waters), direct contact with pets and farm animals, and various predisposing factors proved to play a role in human salmonellosis. The results of the analyses focusing on serotypes suggested that travelling abroad and consumption of eggs are particularly important risk factors for S. Enteritidis infection, while previous intake of antimicrobials was the only risk factor identified for S. Typhimurium. Available studies did not allow for an analysis by region or age group. Figure 2 shows the relative importance of risk factors and routes of exposure to Salmonella, including Salmonella serotypes.
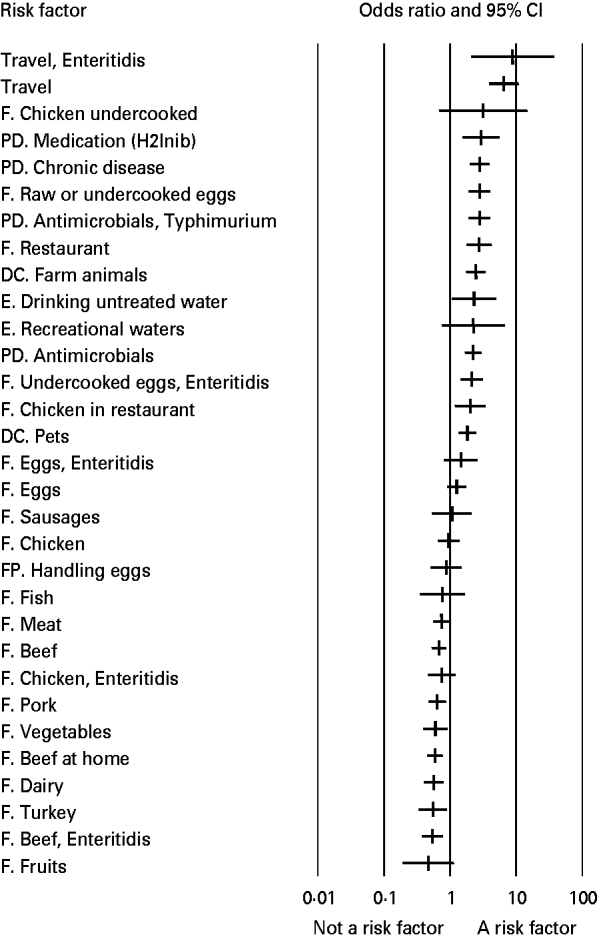
Fig. 2. Relative importance of risk factors for sporadic salmonellosis (odds ratio and 95% CI). F, Food; DC, direct contact with animals; E, environmental transmission; PD, predisposing factors; FP, food Preparation; H2Inib, proton inhibitors medication.
Table 2. The odds ratio (OR) together with the 95% confidence interval (CI) for the risk factors for sporadic salmonellosis
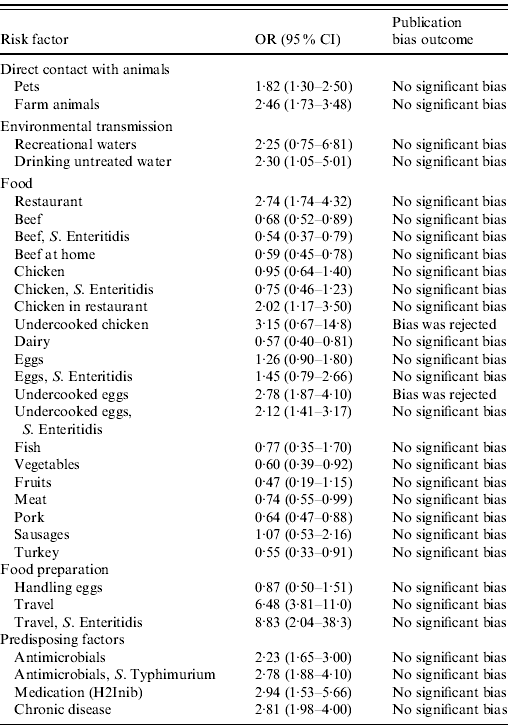
H2Inib, Proton inhibitors medication.
Significant publication bias was not identified in any of the analyses. When the number of studies in the analyses was small, the one-study-removed method frequently indicated the existence of influential studies. For instance, Figure 3 shows the change in the pooled OR for the risk factor eating undercooked chicken when the corresponding study was removed. It shows that removing the study [Reference Pires24] suggests that eating undercooked chicken in a restaurant is a strong risk factor for sporadic salmonellosis (OR 7·09, 95% CI 1·14–43·98). In the analysis of the risk factor eating raw or undercooked eggs, the funnel plot showed a lack of symmetry around the pooled OR (Fig. 4). Duval & Tweedie's trim-and-fill method suggested adding six studies (solid symbols, •) to the left side of the plot to reach complete symmetry, which would result in a shift of the OR with the 95% CI towards the null effect (the black diamond below the x-axis) reducing the effect of eating raw or undercooked eggs as a risk factor for salmonellosis. This was supported by Egger's regression test that indicated a strong association between study size and study effect (intercept=2·82, s.e.=0·55, P<0·01). However, Begg & Mazumdar's correlation test suggested an absence of correlation between study size and study effect (tau=0·3, P=0·1), and the one-study-removed method did not indicate the existence of influential studies. In the analysis of eating undercooked chicken, Duval & Tweedie's trim-and-fill method suggested adding two studies to the right side of the plot to reach complete symmetry (results not shown). This would lead to a shift in the OR from 3·15 (95% CI 0·67–14·8) to 8·4 (95% CI 1·04–67), suggesting that eating undercooked chicken is a risk factor for sporadic salmonellosis. Using the one-study-removed method, an influential study was identified (Fig. 3). In both cases, the existence of publication bias was rejected.
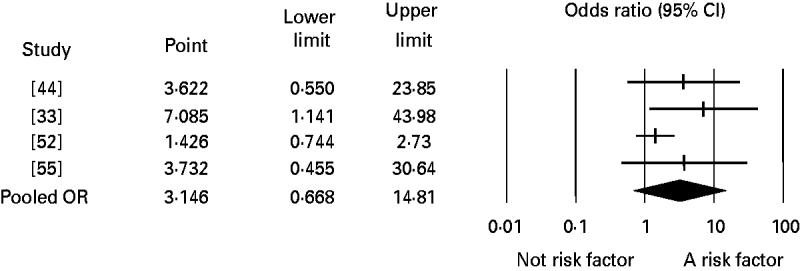
Fig. 3. Forest plot of the change in the pooled odds ratio (OR) of the risk of sporadic salmonellosis following eating undercooked chicken together with the 95% confidence interval (CI), when the corresponding study was removed. The pooled OR represents the effect including the four studies. Study [Reference Pires24] is identified as influential, and when it is removed from the analysis, the pooled OR changes from 3·15 (95% CI 0·67–14·81) to 7·09 (95% CI 1·14–43·98).
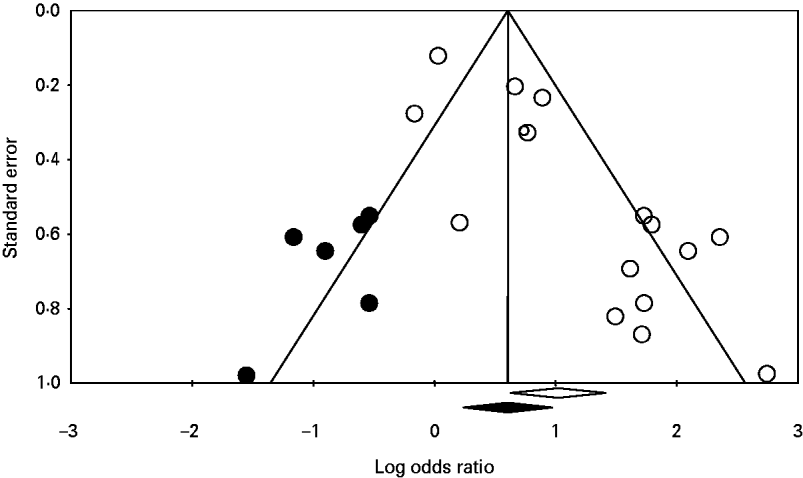
Fig. 4. Funnel plot of the logarithm odds ratio of 16 studies (○) quantifying the effect of eating undercooked or raw eggs on the risk of sporadic salmonellosis. The solid symbols (•) are the potential missing studies according to Duval & Tweedie's trim-and-fill method (if they had existed, the pooled effect would have shifted slightly towards the null effect; the black diamond below the x-axis). The white diamond represents the pooled odds ratio with the 95% confidence interval unadjusted for publication bias.
DISCUSSION
This SR followed a rigorous search strategy to identify all potentially relevant peer-reviewed case-control studies of sporadic salmonellosis. Collected studies were conducted in different countries and time periods (see Appendix), designed with different settings, and sometimes focused on specific age groups within the population. The quality of the studies also varied, and was evaluated on the basis of defined methodological criteria during the formal process of the SR, and not judged on an individual basis by the reviewers.
The risk factors extracted from individual studies were categorized according to main source-classification schemes, and the meta-analyses of collected data were carried out per risk factor stratum, analysing information from all references that assessed the impact of that specific factor on the risk of disease. This categorization implied the harmonization of risk-factor labelling, which may have resulted in loss of information from individual studies. Nonetheless, it allowed for the integration and meta-analysis of results from all collected studies. Additionally, risk factors were included in the analysis only if they were investigated in four or more case-control studies. This criterion resulted in the exclusion of risk factors from the meta-analysis, which may also have resulted in the loss of evidence and potentially biased estimates.
The meta-analysis of sporadic salmonellosis studies highlighted international travel as the most important risk factor for human Salmonella infections. Other source attribution studies published in several countries have identified travel as an important contributor to human salmonellosis, namely in Denmark [Reference Hald25, Reference Pires and Hald26], Sweden [Reference Ekdahl27], and several other European Member States [Reference Pires28]. Travel is expected to be important for the burden of human salmonellosis in countries with relatively low levels of Salmonella in domestic animals and foods and where the population travels frequently, particularly developed countries. Most of the collected studies were conducted in industrialized countries (see Appendix), and all studies included in the meta-analysis for the risk factor travel came from the developed world.
Regarding food products, our analysis only identified eggs eaten undercooked or raw, and chicken consumed in a restaurant as risk factors for salmonellosis. These findings are supported by all Salmonella source attribution studies available in the literature (see e.g. [Reference Pires and Hald26, Reference Havelaar29, Reference Greig and Ravel30]). The fact that our results did not identify other foods as relevant for salmonellosis may be explained by the inclusion of evidence from several studies conducted in a variety of countries throughout the world. On the one hand, studies frequently pose questions to interviewed cases and controls differently, for example asking about the degree of cooking of foods or not, and this may result in different signals in the importance of specific foods for disease. On the other, the contribution of each food to Salmonella infections varies between countries as shown by [Reference Pires24], but the studies included in this meta-analysis did not allow for regional analyses, and thus geographical differences could not be investigated.
Our results suggest that direct contact with live animals (pets and farm animals) and environmental routes, namely drinking and swimming in recreational waters, are important contributors for human salmonellosis. Non-foodborne routes have been previously identified as important routes of transmission of Salmonella (see e.g. [Reference Baker4]), but most source attribution methods applied so far focus solely on foods. Additionally, intervention measures aimed at controlling salmonellosis have traditionally focused on the food chain. Thus, these results provide valuable information for the attribution of human salmonellosis cases and can be used to direct interventions or raise awareness in the population for risk factors of infection.
The meta-analysis included studies investigating risk factors for infections Salmonella spp. and studies focusing on specific serotypes. Some serotypes have more frequently been associated with specific animal sources and foods (e.g. S. Enteritidis with eggs, S. Dublin with cattle and beef), while others may have a broader range of sources (e.g. S. Typhimurium with several animal species and meats), and the weight of each serotype on the overall number of studies could influence the final general results of a meta-analysis. Nonetheless, because the distribution of studies focusing on the most important serotypes and on Salmonella spp. was balanced (see Appendix), we found the potential bias irrelevant.
The analyses by serotype could be conducted only for the most frequent Salmonella serotypes in the human population, S. Enteritidis and S. Typhimurium. The analysis identified travel and eggs as important risk factors for infection with S. Enteritidis, and this is in line with other epidemiological evidence provided by studies focusing on this serotype [Reference Pires and Hald26]. Only predisposing factors were identified as relevant for S. Typhiumrium, which is thought to be a reflection of the low number of studies conducted for this serotype alone.
In the context of source attribution, we did not draw conclusions on factors associated with a statistically significant reduced risk of disease (odds ratio <1). This is justifiable in the light of the potential impact of bias inherent to individual case-control studies (e.g. due to misclassification of exposures due to lack of accuracy of recall), and thus to the final meta-analysis. While this is true for all exposures and all data that originate from patients' and controls' interviews, it is particularly important when making inferences on the protective effect of specific exposures, which may eventually also be routes for infection.
The statistical analysis took into account the potential innate heterogeneity of studies by using the random-effects model [Reference Borenstein17]. Potential factors that could explain the heterogeneity were further investigated using classification. Publication bias results have shown the absence of significant bias. In the analysis of eating raw or undercooked eggs, Duval & Tweedie's trim-and-fill method suggested adding six studies (Fig. 4, solid symbols, •) to the left side of the plot to reach complete symmetry. Adding these missing studies would reduce the effect of eating undercooked or raw eggs as a risk factor for sporadic salmonellosis. The apparently missing studies are small- or medium-sized studies, which increases our suspicions that those studies could have been conducted, but not published, because they do not show interesting results [Reference Halasa18, Reference Ferguson31]. Eating raw or undercooked eggs has been recognized as a source of Salmonella and the confidence limits of the OR before and after correction for publication bias overlap; therefore, the publication bias was rejected. In the analysis of eating undercooked chicken, Duval & Tweedie's trim-and-fill method suggested adding two studies to the right side of the plot to reach complete symmetry (results not shown). Adding these studies would suggest that eating undercooked chicken is a risk factor for salmonellosis. Chicken meat is a known source of Salmonella, as we also show in our current study. Nonetheless, the two missing studies show quite interesting results, and one of them is a large study, which suggests the study would have been published if it had been conducted. The one-study-removed method identified an influential study [Reference Delarocque-Astagneau32], which is expected when there are only few studies available for the analysis [Reference Halasa18]. In this analysis, only four studies were available in which bias could have been observed due to the small sample size. It is recommended that accepting or rejecting a bias should be solely based on rational and biological reasons, and not only based on statistical tests [Reference Halasa18]. Because we could not rationally explain the omission of these two studies given our rigorous search criteria, we rejected the publication bias and deemed it a correct meta-analysis.
We conclude that a SR and meta-analysis of case-control studies are valuable tools to collect and analyse available information on risk factors of sporadic cases of pathogens commonly transmitted through foods. The approach can be applied to a variety of pathogens, and results can be used to assist risk management decisions and identify and prioritize areas for interventions.
ACKNOWLEDGEMENTS
Financial support was provided be the EU Network of Excellence MedVetNet.
APPENDIX Reference, country, subtype, time period, and number of cases and controls interviewed of case-control studies of sporadic salmonellosis collected in the systematic review
DECLARATION OF INTEREST
None.









