INTRODUCTION
The pathogenic potential of non-tuberculous mycobacteria (NTM) has been recognized since the beginning of the last century [Reference Marks and Jenkins1]. Many large series studies have reported that NTM can be attributed to both pulmonary and extrapulmonary diseases, with increasing numbers in both HIV-positive and HIV-negative patients [Reference Marks and Jenkins1–Reference Griffith4]. Of the extrapulmonary diseases caused by NTM, skin and soft-tissue infection (SSTI) was the most common [Reference Henry5–Reference Bodle7].
NTM SSTI is often associated with surgical procedures or trauma [Reference Liao8–Reference Wallace10] and tends to disseminate in hosts with immunosuppression [Reference Wallace, Brown and Onyi11, Reference Ingram12]. Immunosuppression defines patients who had chronic steroid use, immunosuppressive medications, or the following comorbidities, including diabetes mellitus, malignant neoplasms, chronic renal or hepatic disease, rheumatic disease, or AIDS. The clinical and histopathological presentations are diverse and not species specific [Reference Street13]. In addition, no large-scale comparative clinical trials on the treatment of skin diseases caused by these organisms have been reported. Although the American Thoracic Society (ATS) and the British Thoracic Society (BTS) have issued guidelines on the diagnosis and management of NTM infections [Reference Griffith4, 14, 15], the reported treatment regimens varied considerably.
In the current study, the clinical features and outcomes of patients with NTM SSTI in our institute from 2005 to 2008 were analysed together with information obtained from a previous study conducted during 1997–2004 [Reference Liao8]. The results may help to elucidate the spectrum of NTM SSTI.
PATIENTS AND METHODS
Study setting and NTM isolates
A retrospective study of patients with NTM SSTI was conducted for the period from 2005–2008 at the National Taiwan University Hospital (NTUH), a medical centre located in northern Taiwan. Cultures for mycobacteria were performed as described in our previous report [Reference Liao8, Reference Roberts, Koneman, Kim, Balows, Hausler, Herrmann, Isenberg and Shadomy16]. Briefly, the specimen was inoculated onto Middlebrook 7H11 by selective agar (BBL, Becton Dickinson, USA) and the fluorometric BACTEC technique (BACTEC MGIT 960 System; Becton-Dickinson Diagnostic Instrument Systems). Identification of the mycobacterial species was based on standard methods described previously [Reference Roberts, Koneman, Kim, Balows, Hausler, Herrmann, Isenberg and Shadomy16].
Data collection
The medical records of patients with NTM SSTI were reviewed retrospectively with informed consent and approved by the Institutional Review Board of NTUH. Information was collected on demographic characteristics, clinical diseases, immune status, underlying medical conditions, predisposing factors (including prior trauma, marine animal exposure, or recent surgery), histopathological findings, antimicrobial treatment, surgical interventions, and outcomes. Chronic renal disease defined as a serum creatinine level ⩾2 mg/dl, and chronic steroid use as daily use of 20 mg prednisolone for at least 2 weeks. Patients with immunosuppression included those who had chronic steroid use, immunosuppressive medications (chemotherapeutic agents or radiotherapy for malignancy), or followed diabetes mellitus, malignant neoplasms, chronic renal or hepatic disease, rheumatic disease, or AIDS. Our previous data regarding SSTI due to NTM from January 1997 to December 2004 were also reviewed for comparison [Reference Liao8].
Diagnosis of SSTI and osteomyelitis caused by NTM
NTM SSTI was defined as culture of a wound discharge or biopsy specimen of a lesion involving skin, subcutaneous tissue, muscle, synovium or bone yielding NTM in the presence of a compatible syndrome. Two disease groups were categorized: localized skin infection and deep tissue infection. Localized skin infection included cellulitis, mass, nodule, pustules and subcutaneous abscess. Deep tissue infection consisted of tenosynovitis, osteomyelitis, deep neck infection, and paraspinal abscess.
Statistical analysis
Data were analysed using SPSS version 10.0 software (SPSS Inc., USA). Continuous variables were expressed as mean ± standard deviation. Comparisons between continuous variables were analysed using the Wilcoxon rank sum test or independent t test, as appropriate; for ⩾3 groups, Kruskal–Wallis one-way analysis of variance by ranks was used. Comparisons between or in categorical variables were tested by χ2 or Fisher's exact test. A value of P<0·05 was considered significant.
RESULTS
Secular trend of SSTI caused by NTM
During the 4-year study period, 50 patients with culture-proven NTM SSTI were identified and 64 cultures of 80 specimens collected from these 50 patients grew NTM. An average number of 12·5 cases each year was documented with an increasing annual trend in the number of cases. The average annual incidence was 0·62/100 000 outpatients and in-patients. The number of patients infected with rapidly growing mycobacteria (RGM) increased, while the number of patients infected with M. marinum and M. avium-intracellulare complex (MAC) remained the same. From 1997 to 2008, the average incidence of RGM infection was 1·39/100 000 patients. This incidence decreased during 2005–2008 compared to previous years (2·3–2·7/100 000 patients in 1999–2001 and 3·2/100 000 outpatients and in-patients in 2004 [Reference Liao8]).
Clinical characteristics
The clinical characteristics of the 50 patients with NTM SSTI during 2005–2008 are compared to those of patients identified during 1997–2008 in Table 1. The clinical characteristics of SSTIs caused by different NTM species are shown in Table 2. During 1997–2008, patients with M. fortuitum and M. marinum infections were younger than those with MAC (36±16 vs. 55±15, P=0·004; 44±19 vs. 55±15, P=0·056, respectively) and M. abscessus (47±21) infections. The duration from symptom onset to diagnosis was also shorter for patients with M. fortuitum and M. marinum infections, and this difference was especially significant between M. fortuitum and M. abscessus infections (22±27 vs. 80±80 days, P=0·01). Patients with M. fortuitum and M. marinum infections were more likely to have previous invasive procedures or trauma than those with MAC (81·8% and 72·0% vs. 27·8%, P=0·002 and 0·006, respectively) and M. abscessus (54·8%, P>0·05) infections. However, immunosuppressed patients were more likely to have infections of the latter two organisms than the former two (66·7% and 45·2% vs. 9·1% and 24%, P=0·006) (Table 2). M. marinum-related SSTI mostly occurred over extremities (96%) which was less frequent in MAC- and M. abscessus-related SSTIs (50%, P=0·001 and 45·2%, P<0·001, respectively). M. abscessus was more likely to cause localized skin infection (OR 6·8, 95% CI 1·6–29·8, P=0·012) while MAC was more likely to cause deep tissue infection (OR 10, 95% CI 1·0–100·5, P=0·05).
Table 1. Comparison of the characteristics of patients with definite skin and soft-tissue infections at NTUH between 1997–2004 and 2005–2008
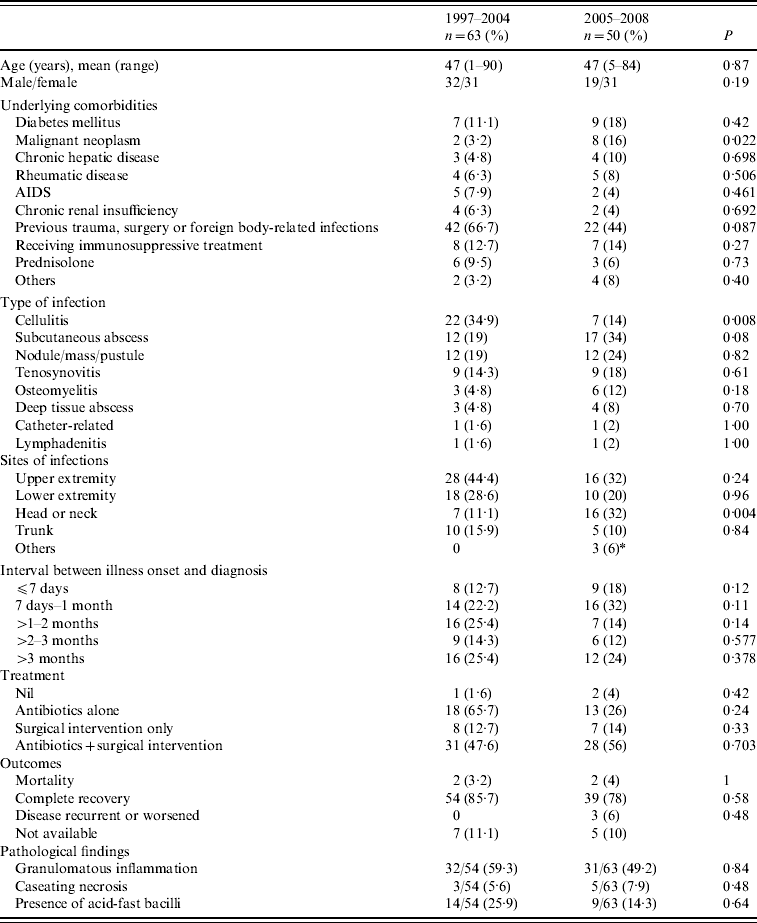
* Includes one case of multifocal osteomyelitis and paraspinal abscess, and two cases of generalized skin lesion and osteomyelitis.
Table 2. Clinical characteristics of 113 patients with skin and soft-tissue infections caused by non-tuberculous mycobacteria according to mycobacterial species
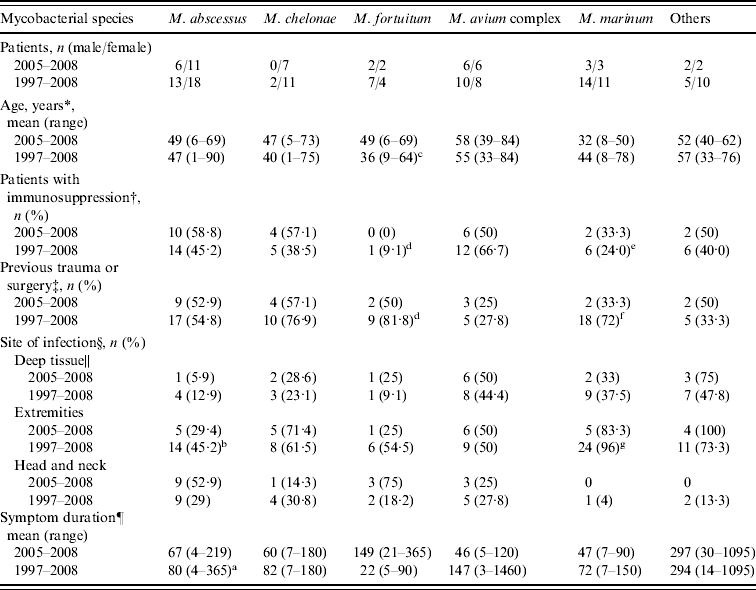
* Significant difference in species, P=0·035.
† Significant difference in species, P=0·006.
‡ Significant difference in species, P=0·007.
§ Significant difference in species, P=0·008.
|| Significant difference in species, P=0·038.
¶ Significant difference in species, P=0·004.
a Compared to M. fortuitum, P=0·01.
b Compared to M. marinum, P<0·001.
c Compared to M. avium-intracellulare complex, P=0·004.
d Compared to M. avium-intracellulare complex, P=0·002.
e Compared to M. avium-intracellulare complex, P=0·014.
f Compared to M. avium-intracellulare complex, P=0·006.
g Compared to M. avium-intracellulare complex, P=0·001.
Treatment and outcomes
The mean treatment duration of SSTI during 2005–2008 was 225·3±249·9 days. Two patients died of causes unrelated to NTM infection. For RGM infections, clarithromycin and fluoroquinolones were most the often administered, to 24 (48%) and 15 (30%) patients respectively, 14 of whom received a combination of both drugs. Amikacin and imipenem were given to 11 (22%) patients with RGM SSTI.
The type of treatment is summarized in Table 1. Specific medications used and the response of SSTI caused by different NTMs during 1997 to 2008 are summarized in Table 3. The total treatment duration was 202·4±207·3 days. Four (3·5%) patients died of causes other than NTM infections. Of the 65·5% of patients who underwent surgical debridement, 69·8% had localized cutaneous disease while 65·5% had disease over the extremities. Of the patients who received intravenous antibiotic therapy, 55·6% and 27·8% had disease located over the head/neck and trunk, respectively. In contrast, 72·6% of patients with infections over the extremities were treated with oral medication (P<0·001). Of the 15 patients who received surgical debridement, one had finger tenosynovitis and the rest had skin nodules or ulcers. All were cured without recurrence. The treatment duration was shortest in patients who received surgery only (55·5±83·8, range 5–180 days) and longest in patients who received medical treatment only (227·8±312, range 28–1400 days, P=0·033). Patients with localized cutaneous infections had a shorter treatment course (161·7±121·2, range 5–540 days) than those with deep tissue infections (287·7±307·8, range 7–1400 days, P=0·026).
Table 3. Treatment and response of 113 patients with skin and soft-tissue infections caused by non-tuberculous mycobacteria according to mycobacterial species
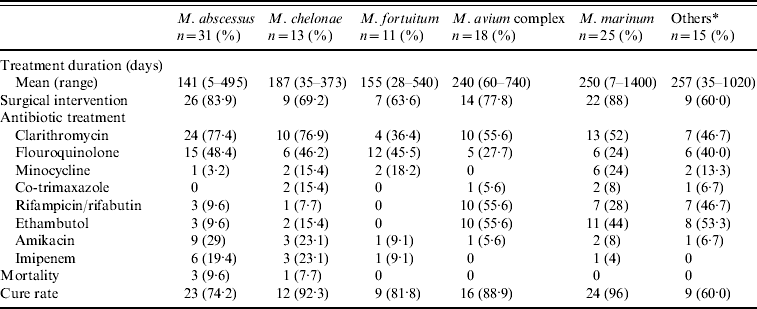
* Includes M. kansasii (n=5), M. haemophilum (n=2), M. gordonae (n=3), M. scrofulaceum (n=1), M. flavescens (n=1), M. arupense (n=1), M. terrae triviale complex (n=1), unidentified NTM (n=1).
Three patients had recurrent NTM diseases (Table 4). Case 1, a case of diabetes mellitus and sarcoidosis, had a positive culture for M. gordonae from nose biopsy. The lesion improved under initial treatment with rifampicinn, ethambutol and ciprofloxacin for 4 months but worsened 7 months later and repeated biopsy culture yielded MAC. The lesion resolved after discontinuation of systemic steroid combined with shifting of therapy to rifampicin, ethambutol, moxifloxacin and clarithromycin. Case 2 was treated with clarithromycin, ciprofloxacin and doxycyline for disseminated M. fortuitum infection for 1 year. However, para-spinal abscess and lumbar osteomyelitis caused by M. marinum was found 6 months after anti-NTM medication was stopped. The osteomyelitis persisted despite prolonged treatment study and the patient was finally lost to follow-up. Case 3 had persistent CD4 count <250/μl following the diagnosis of HIV infection. A 1-year treatment plan with amikacin, ethambutol, clarithromycin, ciprofloxacin and rifabutin was initiated to treat MAC bacteraemia after culture was initially negative. However, disseminated MAC infection was found in cultures several months later and therapy was switched to moxifloxacin, rifabutin and clarithromycin for 6 months. Five months later, MAC grew from cultures of the lower leg skin abscess and rifampicin, clarithromycin and ethambutol were restarted again. This patient's CD4 count progressively increased under HAART and was 1621/μl at the time MAC treatment was completed.
Table 4. Characteristics of patients with recurrent skin and soft tissue infection caused by non-tuberculous mycobacteria
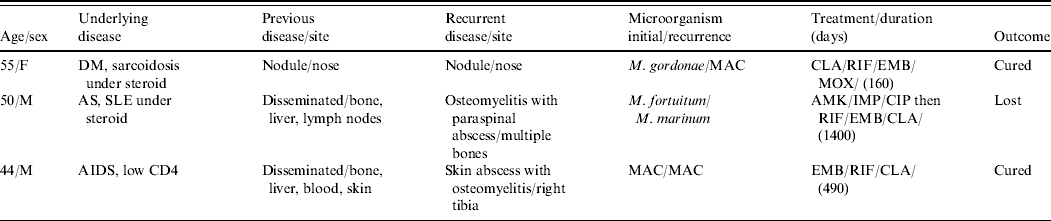
DM, Diabetes mellitus; AS, ankylosing spondylitis; SLE, systemic lupus erythematosus; AIDS, acquired immunodeficiency syndrome; MAC, M. avium-intracellulare complex; CLA, clarithromycin; RIF, rifampicin; EMB, ethambutol; MOX, moxifloxacin; AMK, amikacin; IMP, imipenem; CIP, ciprofloxacin; MTB, Mycobacteria tuberculosis.
Pathological findings
Forty-one (82%) patients had at least one biopsy study during 2005–2008. Patients with immunosuppression were less likely to develop granuloma at their infection sites (38·9% vs. 73·9%, P=0·031 during 2005–2008; 40% vs. 60·1%, P=0·019 during 1997–2008) and were older (40±21·5 vs. 55·3±13·2, P=0·007) than patients without immunosuppression. Granuloma was also more often found in M. marinum-infected tissue (78·3%). Positive acid-fast stain was observed slightly more often in biopsy specimens from patients with immunosuppression (29·4% vs. 18·2%, P=0·465).
Differences in NTM SSTIs between 1997–2004 and 2005–2008
The interval between the recorded onset of symptoms and the day of culture-confirmed diagnosis was 87·7±163·5 days (range 4–1095 days) in 2005–2008, which was shorter than that for 1997–2004 (average 131·8±236·8). The percentage of immunosuppressed patients increased in the latter period from 31·7% to 48%, and fewer patients had previous experience of trauma, surgery or aquatic animal exposure (44% vs. 66·7%). RGM remained the most common pathogens but MAC-related infection increased and infection due to M. marinum decreased in the latter period (24% vs. 9·5%, 12% vs. 30·2%, respectively). Amikacin and imipenem use increased from 6·3% in 1997–2004 to 28% in 2005–2008 (P=0·003), while clarithromycin use increased from 48% to 74% (P=0·007).
DISCUSSION
This study investigated the clinical, microbiological, pathological characteristics and outcomes of 113 patients with NTM SSTI from 1997 to 2008. The average incidence was 1·39/100 000 outpatients and in-patients, and declined during the last 4 years (2005–2008). In an epidemiological study conducted in an urban community in the UK, 27% of 72 non-HIV patients with NTM disease had SSTIs [Reference Henry5]. This incidence was about 0·11–1·4/100 000. In another study in the USA, the incidence of NTM disease of any body site was 2·7/100 000 non-HIV patients and 18% of these patients had SSTIs [Reference Bodle7]. However, it is difficult to compare our findings with previous studies due to the use of different epidemiological methods.
In a series of 125 cases of RGM infections, M. fortuitum was the most frequently isolated pathogen [Reference Wallace10]. In two series of patients with cutaneous infection, M. marinum was the predominant isolated organism [Reference Street13, Reference Dodiuk-Gad17]. In the current study, the most common microorganism was M. marinum from 1997 to 2004, and M. abscessus from 2005 to 2008. Although this study found a decreasing trend in cases of NTM SSTIs in patients with previous trauma experience and an increasing trend in cases in immunosuppressed patients, whether these trends were related to changes in the dominant causative NTM species remains unclear.
Similar to previous studies, the ages of our patients with M. fortuitum and M. marinum SSTIs were younger, and were often associated with previous invasive procedures or trauma, and less often associated with immunosuppressive comorbidities than those in patients with M. chelonae, M. abscessus, or MAC SSTIs [Reference Dodiuk-Gad17–Reference Bartralot19]. Interestingly, we found that M. abscessus more often caused localized skin lesion and carried a higher risk of deep tissue involvement than MAC SSTIs. This could not be explained by the difference in percentages of immunosuppressed patients infected by the two species, since over half of the patients with M. chelonae and M. abscessus infections were immunosuppressed in this study, a similar percentage to those infected with MAC, nor was there any significant difference in the percentage of patients with previous trauma. Immunosuppressed patients with M. abscessus or M. chelonae SSTIs have been reported to present multiple skin lesions (>5) and disseminated skin lesions more often than those without any underlying diseases [Reference Wallace, Brown and Onyi11, Reference Ingram12, Reference Uslan18]. In previous studies, MAC mostly occurred in persons infected with HIV [Reference Horsburgh20–Reference Reed22]. However, an increased incidence of MAC infection in non-HIV patients has been reported in several recent studies [Reference Henry5, Reference Bodle7], mainly in children with lung and cervical lymphadenitis.
The type and duration of antimicrobial treatment for NTM-related infections remains poorly established [Reference Griffith4, 14, 15, Reference Wallace23, Reference Wagner and Young24]. The treatment regimen for non-pulmonary disease caused by RGM (M. abscessus, M. chelonae, M. fortuitum) was suggested to be based on in vitro susceptibilities. For M. abscessus disease, a macrolide-based regimen was recommended. Surgical debridement may also be an important element of successful therapy [Reference Griffith4]. In our previous study, amikacin was active against almost all RGM isolates [Reference Yang25]. Clarithromycin was usually active against M. abscessus (79–91% susceptible) and M. fortuitum (65% susceptible). M. fortuitum was highly susceptible to ciprofloxacin (62–83·3%) and imipenem (61–100%). However, the susceptibility of M. abscessus to imipenem varied widely in our previous studies (from 18% to 82%) [Reference Liao8, Reference Yang25]. We treated our patients with RGM SSTI based on the susceptibility patterns determined in our previous study [Reference Yang25], and the resulting cure rate was 72–93%. These previous studies and the current study support the view that surgical debridement alone might be enough for patients with disease localized to skin and without any underlying comorbidities.
The combination of rifampicin and ethambutol or monotherapy with doxycycline, minocycline, or trimethorpim–sulfamethoxazole (co-trimoxazole) has been suggested for the treatment of M. marinum SSTI [Reference Wagner and Young24–Reference Cummins, Delacerda and Tausk28]. In vitro and in vivo susceptibility studies of M. marinum to clarithromycin have been previously reported [Reference Alloway, Evangelisti and Sartin29–Reference Aubry31]. Most of our patients with M. marinum SSTIs were treated with clarithromycin with or without ethambutol. Nearly half of our patients with MAC SSTIs received macrolide, rifampicin and ethambutol with or without surgical intervention as suggested by ATS/BTS guidelines [Reference Griffith4, 14, 15]. Most of our patients were cured after 6–9 months of medical treatment except for HIV patients with low CD4 counts, which is consistent with the results of an animal study in mice which showed that CD4 cells could protect against dissemination of MAC from the intestine [Reference Petrofsky and Bermudez32]. In this study, one HIV-infected patient had recurrent MAC infection; however, it was not determined whether these MAC isolates had different serotypes or pathogenicity as reported in other studies [Reference Julander33, Reference Ohkusu34].
In addition, previous studies of mycobacterial DNA in the cutaneous lesions of patients with sarcoidosis identified M. avium in 20–30% of patients [Reference Ning35, Reference El-Zaatari36]. Furthermore, M. avium subspecies paratuberculosis (MAP), an emerging pathogen, has been suggested to play a possible role in the pathogenesis of sarcoidosis and Crohn's disease [Reference Greenstien and Collins37–Reference Naser, Ghobrial and Romero39]. The agents that are referred to as anti-inflammatories, immunomodulators and immunosuppressants all cause dose-dependent inhibition of MAP in culture. The more potent anti-inflammatory agents, notably methotrexate and 6-mercaptopurine, caused dose-dependent inhibition of all mycobacterial strains (M. avium subspecies avium and M. tuberculosis complex) in previous studies [Reference Greenstien and Collins37, Reference Greenstein38]. Although MAP was not identified in isolates from this sample of patients with NTM SSTI, further study using advanced laboratory methods is needed to better understand this uncommon aetiology.
In conclusion, the clinical presentation and treatment varied in SSTIs caused by different NTM species in this study. The recurrence of NTM SSTI is often related to immunosuppression.
DECLARATION OF INTEREST
None.






