INTRODUCTION
Around 5·0–38·0% of respiratory virus infections develop into acute lower respiratory (tract) infections (ALRIs) leading to hospitalization and mortality [Reference Wright1–Reference Monto and Sullivan4]. Thompson et al. [Reference Thompson5] estimated deaths associated with influenza A/B and respiratory syncytial virus (RSV) between 1976 and 1999 in the USA. They reported that between 1990 and 1999 there were an average of 8097 (s.d. = 3084) influenza A/B/pneumonia-associated deaths and 2707 (s.d. = 196) RSV/pneumonia-associated deaths annually, with 90·0% of influenza- and 78·0% of RSV-associated deaths occurring in the elderly aged ⩾65 years. In the UK a study by Nicholson [Reference Nicholson6] estimated that between 1975 and 1990 there were between 6244 (s.d. = 1416) and 29 646 (s.d. = 6723) influenza-related deaths annually. Two recent meta-analyses on influenza and RSV in children aged ⩽5 years [Reference Nair7, Reference Nair8] estimated that globally, 13·0% of all children with influenza infection developed ALRI – equivalent to 20 million [95% confidence interval (CI) 13–32 million] ALRI cases per year, and that in 2008, influenza-associated ALRI caused between 28 000 and 111 500 deaths in children aged ⩽5 years. Nair et al. [Reference Nair8] reported that 22·0% of all ALRIs were associated with RSV (incidence 59·1/1000 children in developing countries and 24·0/1000 children in developed countries, respectively) – equivalent to a global health burden of 33·8 million new cases each year; of which 3·4 million (9·0%) cases required hospitalization, and 66 000–199 000 died. An earlier review by Williams et al. [Reference Williams9] suggested that globally, ALRIs were responsible for 1·9 million (95% CI 1·6–2·2 million) deaths in children aged ⩽5 years.
Some studies have reported an association between co-infection and severe disease outcome [Reference Richard10–Reference Marcone15]; however, a number of other studies have reported no significant differences in disease outcome between single or multiple virus infections [Reference Schnepf16–Reference Bicer18]. Our earlier study [Reference Goka19] investigated the association between co-infection between pandemic influenza A(H1N1)pdm09 (Flu Apdm09), seasonal influenza A virus (SeasFlu A) and other respiratory viruses and risk of hospitalization and mortality. The present study compares the disease outcome in single RSV, rhinovirus (RV), adenovirus (AdV), human metapneumovirus (hMPV), human parainfluenza virus types 1–3 (hPIV1–3), influenza B (Flu B), Flu Apdm09, and SeasFlu A viruses. It also investigates the association between single and multiple infections, in general, and disease outcome. Knowledge on single infections will help determine the relative importance of these respiratory viruses in causing acute respiratory infections requiring hospitalization or resulting in death, whereas an understanding of disease outcome in co-infections may help generate information needed for patient management, direct research efforts towards development of multi-targeted diagnostic tests, integrated vaccine, and combined treatment for all respiratory viruses.
METHODOLOGY
Study design and setting
This descriptive cross-sectional study retrospectively analysed the results of respiratory virus samples of patients aged 0–105 years that were received and tested for ten respiratory virus infections: Flu Apdm09, SeasFlu A viruses, e.g. H3N2 (SeasFlu A), Flu B, RSV, RV, AdV, hMPV, and hPIV1–3, at Manchester Microbiology Partnership Laboratory (MMPL) between January 2007 and June 2012. The samples were from outpatients, hospitalized patients, or patients who had died, and they originated from medical centres, surgeries and hospitals located in the North West region of England, an area serving a population of over 7 million people [20]. In total 30 975 samples were received at the MMPL between January 2007 and June 2012, of which 35·9% (11 112) were from children aged ⩽5 years and 64·1% (19 863) were from patients aged > 5 years [mean ± s.d. 24·4 ± 24·1 years, median 20·0 (25–75 percentiles 0·8–41·0 years)]; differences in these proportions were statistically significant (P < 0·0001).
Virus identification, inclusion and exclusion criteria
The types of samples that were tested for respiratory virus infection included: nose and throat swabs (VNT), nasopharyngeal aspirates (VNPA), and swabs from other parts of the body (VSW). Details of the laboratory protocols used to test for the viruses have been published elsewhere [Reference Goka19]. Briefly, well validated in-house real-time polymerase chain reaction (RT–PCR) assays were used for the identification of influenza and other respiratory virus infections. In a second assay [Reference Ellis21], RT–PCR was used to further type the Flu Apdm09 virus.
The majority, but not all samples were tested for all respiratory viruses as tests were conducted according to the physicians' request. All samples received at the MMPL and tested for respiratory virus infections during the study period were eligible for inclusion. Samples that did not have test results because of failure of PCR or insufficient sample, or had details of the patient's outcome status [i.e. hospitalization to the general ward or intensive care unit (ICU) or died] missing, were excluded.
Statistical analyses
Descriptive statistics were calculated to explore the distribution of single, dual and multiple respiratory virus infections by age, gender and season. Pearson's χ 2 test was used on this categorical data at the significance level of P = 0·05. Multiple logistic models, controlling for age, season, gender and an interaction factor between age and co-infection, were used to measure the association between single, dual, and multiple respiratory virus infections and risk of admission to a general ward, admission to an ICU or death. First, a univariate logistic model was calculated and then a second covariate was added to the model up to the last, and the best model chosen. The significance of each covariate was assessed using the likelihood-ratio test. Association was measured using odds ratios (ORs) and 95% confidence intervals (CIs) at significance of P = 0·05. All analyses were conducted using Stata software version 11 (StataCorp, USA).
Ethical and research and development approval
Ethical approval for this study was granted by the Greater Manchester NHS Ethics Committee (ref. 11/NW⁄0698) and the University of Manchester Research Ethics Office. Research and Development approval was obtained from the Central Manchester Universities Hospitals NHS Foundation Trust (ref. R01835).
RESULTS
Respiratory virus infections
During the six years of the study, 30 975 samples were tested and 11 715 (37·8%) were positive for one or more respiratory viruses of which, dual or multiple infections occurred in 10·4% (1214/11 715), and single infections occurred in 89% (10 501/11 715). Of the dual infections, the most common combination was RV/RSV (30·2%, 367 of all co-infections), followed by RV/AdV (13·9%, 169), RSV/AdV (7·2%, 88), RV/hPIV3 (5·2%, 63), RV/hMPV (5·0%, 61), AdV/hMPV (2·1%, 25), RSV/hMPV (1·5%, 18), AdV/hPIV3 (1·4%, 17) and RV/hPIV1 (1·3%, 16) (Table 1). For triple and multiple infections, RVs and RSVs predominated with the RV/RSV/AdV triple infection comprising 32 (2·7%) of all co-infections, followed by a triple infection involving the three human parainfluenza viruses hPIV1/hPIV2/hPIV3 (16, 1·3%) and multiple infections of RV/hPIV1/hPIV2/hPIV3 and RSV/hPIV1/hPIV2/hPIV3–13 (1·1%). In total RV was involved in 844/1214 co-infections (69·5%) making them the most common co-infecting virus. The pattern of co-infections between the Flu Apdm09 and SeasFlu A viruses and other respiratory viruses have been presented elsewhere [Reference Goka19].
Table 1. Patterns of single, dual and multiple infections between respiratory viruses
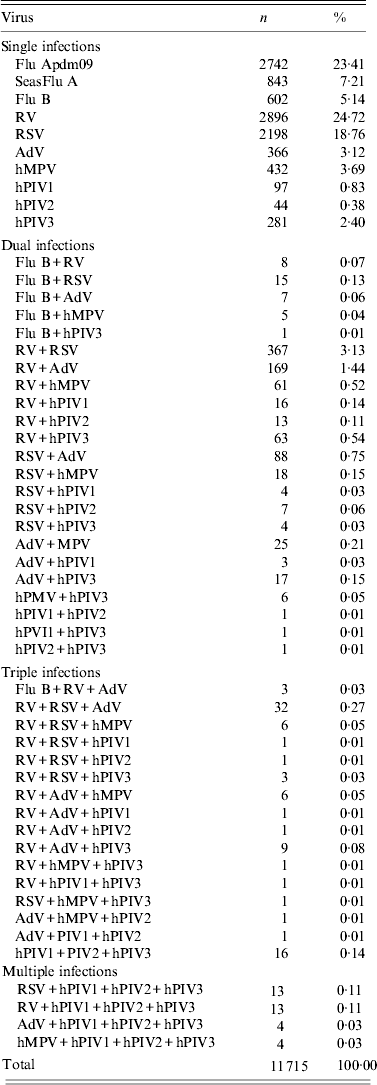
Flu Apdm09, Influenza A(H1N1)pdm09; SeasFlu A, seasonal influenza A virus; Flu B, influenza B virus; RSV, respiratory syncytial virus; RV, rhinovirus; AdV, adenovirus; hMPV, human metapneumovirus; hPIV 1–3, parainfluenza virus types 1–3.
Patterns of co-infections between Flu Apdm09 and SeasFlu A viruses with other respiratory viruses have been presented elsewhere [Reference Goka19].
The percentage of each observed infection is from the total number of all identified virus infections (11 715).
Demographic and other factors associated with single or multiple infections
Age and sex
The majority of patients (51·2%, 6065/11 715) positive for one or more virus were children aged ⩽5 years (mean ± s.d. 16·5 ± 21·4, range 0–99, median 3·0 years; 25–75 percentiles 0·42–28·0 years) compared to 26·6% (4579/17 248) and 23·3% (468/2012) of children aged ⩽5 years in PCR-negative and excluded patients; these differences were statistically significant (χ 2 = 1900, P = <0·0001, and χ 2 = 559·6, P < 0·0001, respectively). The distribution of single and multiple infections by age and gender is given in Table 2a . Again children had a higher risk of having a mixed infection, with 83·5% (1014/1214) of mixed infections occurring in those aged ⩽5 years. Similar proportions were observed for specific respiratory virus infections. However, apart from RV, Flu B and hMPV, the attack rates for the other respiratory viruses did not significantly differ by sex.
Table 2a. Demographic and other characteristics of single and multiple respiratory virus infections in North West England, 2007–2012
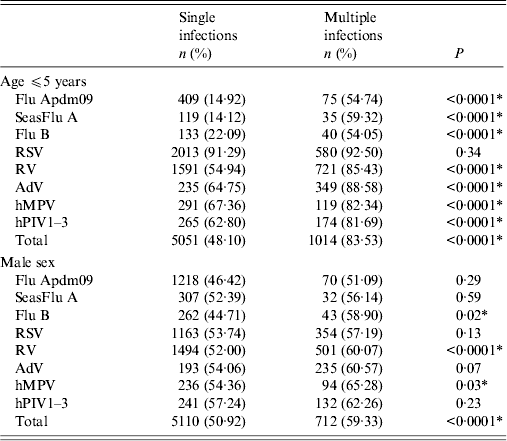
Flu Apdm09, Influenza A(H1N1)pdm09; SeasFlu A, seasonal influenza A virus; Flu B, influenza B virus; RSV, respiratory syncytial virus; RV, rhinovirus; AdV, adenovirus; hMPV, human metapneumovirus; hPIV 1–3, parainfluenza virus types 1–3.
Distribution of single infections compared to multiple infections by age and sex. For each virus, the total number of single or multiple infections were evaluated, i.e. how many single infections occurred in children aged ⩽5 years and how many multiple infections occurred in this age group. For example, of the 3740 RV infections, 2896 were single infections and 844 were mixed infections. Of the 2896 single RV infections, 1591 (54·9%) were aged ⩽5 years, and of the 844 mixed infections 721 (85·4%) were aged ⩽5 years.
Pearson's χ 2 was used to measure the differences in frequency of single and multiple infections. The χ 2 statistic is a comparison between the percentages of children aged ⩽5 years with single infections (54·9%) and with mixed infections (85·4%), for RV for example.
* The P value was statistically significant at α<0·05.
Table 2b. Respiratory viruses identified in each season North West England 2007–2012
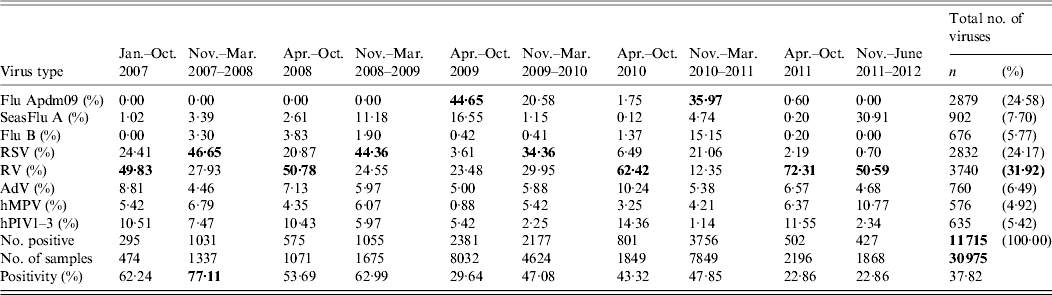
Flu Apdm09, Influenza A(H1N1)pdm09; SeasFlu A, seasonal influenza A virus; Flu B, influenza B virus; RSV, respiratory syncytial virus; RV, rhinovirus; AdV, adenovirus; hMPV, human metapneumovirus; hPIV1–3, human parainfluenza virus types 1–3.
Some of the samples had more than one virus identified from them. Not all samples were tested for all viruses, positivity is overall positive rate. For each period, percentage of each virus is percentage from the total number of respiratory viruses identified in that period.
Bold values indicate the type of virus that predominated in each period.
Seasonality
There was a fourfold increase in the number of samples received at the MMPL during the first and third waves of the influenza pandemic, April–October 2009 and November 2010–March 2011 [25·9% (8032/30 975) and 25·3% (7849/30 975)], compared to the other periods of the study (range 474–2196 in pre-, inter-, and post-pandemic periods). Correspondingly, the majority of the respiratory viruses were identified during the pandemic waves [20·3% (2831/11 715) and 32·1% (3756/11 715)] (Table 2b ). The type of virus that predominated each period also differed; influenza viruses were predominant during the first and third waves whereas RV and RSV predominated in the pre-, inter-, and post-pandemic periods (RV mainly in summer and RSV mainly in winter). However overall RV was the most predominant virus comprising 31·9% (3740/11 715) of all viruses, followed by Flu A(H1N1)pdm09 (24·6%) and RSV (24·2%).
Single and multiple infections and risk of hospitalization to a general ward, or admission to an ICU and death
Of the 10 501 patients with single respiratory virus infections, 1738 (16·6%) were seen as outpatients, 8009 (76·3%) were admitted to a general ward, 530 (5·1%) were admitted to an ICU, and 224 (2·1%) died. On the other hand, out of the 1214 patients who had co-infections, 147 (12·2%) were seen as outpatients, 992 (81·7%) were admitted to a general ward, 57 (4·7%) were admitted to an ICU, and 18 (1·5%) died. In stratified analysis multiple infection in children aged ⩽5 years was associated with an increased risk of admission to a general ward (OR 1·32, 95% CI 1·10–1·63, P = 0·01). In a multiple logistic model controlling for age and season, the risk was slightly higher (OR 1·43, 95% CI 1·20–1·71, P < 0·0001) (Table 3).
Table 3. Risk of admission to a general ward in single and multiple respiratory virus infections
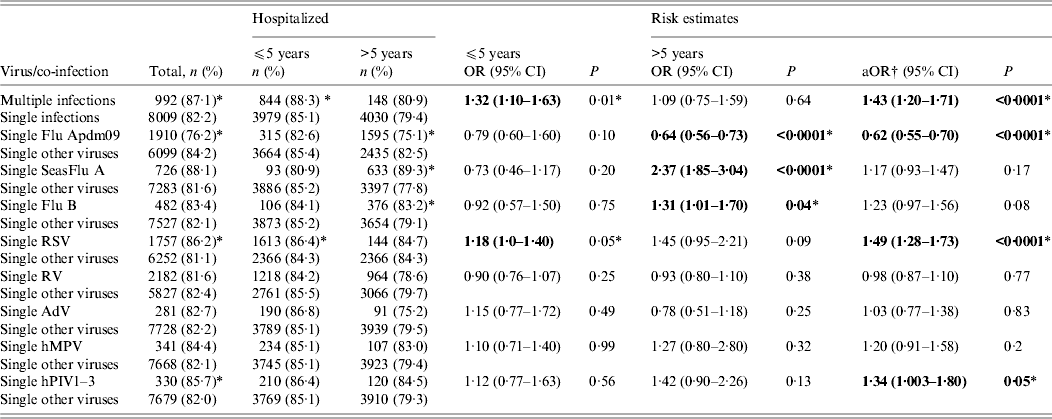
OR, Odds ratio; aOR, adjusted odds ratio; CI, confidence interval; Flu Apdm09, Influenza A(H1N1)pdm09; SeasFlu A, seasonal influenza A virus; Flu B, influenza B virus; RSV, respiratory syncytial virus; RV, rhinovirus; AdV, adenovirus; hMPV, human metapneumovirus; hPIV1–3, human parainfluenza virus types 1–3.
In comparisons between single infections, the risk of admission to a general ward for the primary virus (e.g. RSV single infections) was compared to the risk in all other respiratory virus single infections as a baseline. On the other hand, in crude analysis of risk in single vs. multiple infections, the risk in single infections was used as the baseline.
* The P value was statistically significant at α<0·05. Statistically significant values are highlighted in bold.
† Adjusted for age group and season.
We wished to discover whether the disease outcome in specific single respiratory virus infections was less, or more severe, compared to other single respiratory virus infections. In this analysis, stratified analysis showed that SeasFlu A virus, and Flu B virus increased the risk of admission to a general ward for young children and adults aged >5 years (Flu A: OR 2·37, 95% CI 1·85–3·04, P < 0·0001; Flu B: OR 1·31, 95% CI 1·01–1·70, P = 0·04), whereas RSV increased risk of admission to a general ward in children aged ⩽5 years (OR 1·18, 95% CI 1·03–1·40, P = 0·05). In a multiple logistic model, controlling for age and season, RSV and hPIV1–3 single infections were associated with a significant increase in risk of admission to a general ward compared to other single respiratory virus infections (OR 1·49, 95% CI 1·28–1·73, P < 0·0001 and OR 1·34, 95% CI 1·003–1·80, P = 0·05, respectively).
Regarding risk of admission to an ICU/death, in general, multiple infection was associated with an increase in the risk of admission to an ICU or dying (OR 1·15, 95% CI 0·86–1·55), compared to single infection but this was not statistically significant (P = 0·34). For specific viruses, RSV and hPIV3 single infections were associated with an increased risk of admission to an ICU or dying than other single respiratory virus infections (OR 1·51, 95% CI 1·20–2·0, P = 0·001 and OR 1·60, 95% CI 1·02–2·40, P = 0·04, respectively) (Table 4). Stratified analysis indicated that the risk of admission to an ICU/death associated with RSV and hPIV were only significant in patients aged >5 years (RSV: OR 2·42, 95% CI 1·36–4·33, P = 0·003; hPIV1–3: OR 1·92, 95% CI 1·10–3·74, P = 0·05).
Table 4. Risk of admission to ICU/death in single and multiple respiratory virus infections
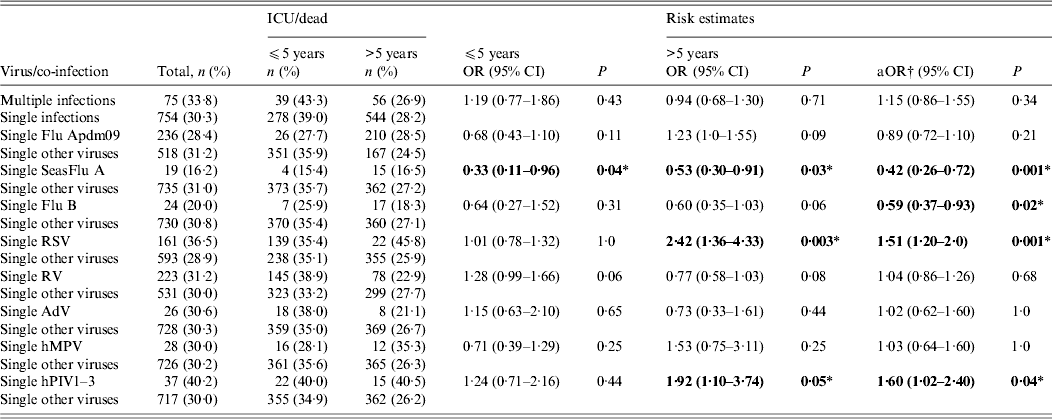
OR, Odds ratio; aOR, adjusted odds ratio; CI, confidence interval; Flu Apdm09, Influenza A(H1N1)pdm09; SeasFlu A, seasonal influenza A virus; Flu B, influenza B virus; RSV, respiratory syncytial virus; RV, rhinovirus; AdV, adenovirus; hMPV, human metapneumovirus; hPIV1–3, human parainfluenza virus types 1–3.
In comparisons between single infections, the risk of admission to an ICU or death for the primary virus (e.g. RSV single infections) was compared to the risk in all other respiratory virus single infections as a baseline. On the other hand, in crude analysis of risk in single vs. multiple infections, the risk in single infections was used as the baseline.
* The P value was statistically significant at α<0·05. Statistically significant values are highlighted in bold.
† Adjusted for age group and season.
In the multivariate logistic regression, separate models controlling for age group, season, sex, and an interaction factor of age and co-infection were employed. After assessment using the likelihood-ratio test, it was observed that models that included both age and season were the best, probably because the distribution of single and multiple virus infections differed statistically by age and season. For example, the result of likelihood test for a logistic model measuring association between single RV infection and risk of admission to a general ward (one without covariates, one with age group, and another with age group and season included) were: χ 2 = 3·74, P = 0·05 and χ 2 = 7·7, P = 0·02, respectively, indicating that both age and season were significant variables.
DISCUSSION AND CONCLUSION
In general, patients with dual or mixed respiratory virus infections had an increased risk of being admitted to a general ward compared to those with single infections. However, patients with RSV and hPIV1–3 single infections had a higher risk of being admitted to a general ward, to an ICU or dying. The finding that co-infections increase disease severity is in agreement with several other studies [Reference Richard10–Reference Semple12] that have reported an association between co-infections and disease severity. Drews et al. [Reference Drews22] showed that co-infection increased the risk of admission to a general ward threefold, while Richard et al. [Reference Richard10] and Do et al. [Reference Do23] also found that co-infection increased the risk of ICU admission by threefold (OR 2·7, 95% CI 1·2–6·2 and OR 3·0, 95% CI 1·6–5·6, respectively). The latter finding is in agreement with other studies [Reference Nair7–Reference Williams9, Reference Schnepf16–Reference Bicer18, Reference Banerji24–Reference Weber, Mulholland and Greenwood26] that observed severe disease in single respiratory virus infections. In general, multiple respiratory virus infections caused more severe disease in children aged ⩽5 years whereas for single virus infections; RSV accounted for severe disease in children (general ward) aged ⩽5 years and in patients (ICU/death) aged >5 years, whereas SeasFlu A virus, Flu B, and hPIV1–3 had higher impact on patients aged >5 years.
In this study, influenza A(H1N1)pdm09 was predominant during the first and third waves of the influenza pandemic, whereas RV and RSV were the main viruses in summer and winter periods of the pre- and post-pandemic periods (Table 2b ). However, considering the entire study period, RV was the most common virus (31·9%, 3740/11 715), followed by the Flu Apdm09 virus and RSV. We also observed that the majority of single and multiple respiratory virus infections were in children aged ⩽5 years (Table 2a ). A study by the European Paediatric Influenza Analysis (EPIA) project [Reference Paget27] on the activity of respiratory virus between 2002 and 2008 in England, reported influenza A and RSV as the predominant viruses. Elsewhere, epidemiological studies from across the globe [Reference Nair7, Reference Nair8, Reference Weber, Mulholland and Greenwood26, Reference Bueving28–Reference Gessner, Shindo and Briand30] have identified RSV as the most common virus in patients visiting outpatient clinics with influenza-like illnesses or hospitalized with acute respiratory (tract) infection or from the community, followed by RV, and influenza A viruses.
RV, RSV and AdV caused the highest co-infection burden [occurring in 69·5% (844/1214), 52·2% (634/1214) and 32·5% (394/1214) of all co-infections, respectively]. Of the others, RV was detected in 367 co-infections with RSV and 169 with AdV (Table 1). Our findings agree with some studies [Reference Echenique14, Reference Schnepf16, Reference Bicer18] but differ with others [Reference Marcone15, Reference Schildgen31]. The high number of RV and RSV co-infections could be because they circulated all year round (Table 2b : RV mainly in summer, but a good number were also identified during winter and vice versa for RSV). Further, hPIV1–3 were the fourth most important co-infecting virus, involved in 17·6% (213/1214) of co-infections and most of the mixed infections. This cannot be explained by seasonal variations as hPIV1–3 circulated predominantly in spring and summer. Monto & Sullivan [Reference Monto and Sullivan4] and Macorne et al. [Reference Marcone15] also found hPIV1–3 predominating in spring and summer whereas Bicer at al. [Reference Bicer18] reported that hPIVs circulate all year round. More research is needed to understand the biomedical processes that favour these co-infections.
A limitation of this study is that we did not control for other covariates known to affect disease outcome; including patient's immune status and comorbidities, and genetic mutations occurring in influenza A viruses and this should be borne in mind when interpreting the results. This study included both children and adults; however, children aged ⩽5 years differ considerably with adults in their exposure to infections, immunology and anatomy. The odds ratios in different age groups were different in themselves and also differed with the crude odds ratios which suggest effect modification by age; however, we did not have serological data to ascertain the patients' immune status. We reported both stratum-specific and adjusted odds ratios because adjusted odds ratios differed with crude odds ratios by more than 10%, suggesting age was also a confounder. Moreover, samples were not tested for human bocavirus and coronaviruses which are known to co-infect with other respiratory viruses [Reference Schildgen31, Reference Zhenqiang, Formenty and Roth32].
It is well understood that genetic mutations may affect the severity of respiratory viruses. For example, the severity of influenza viruses may be influenced by genetic mutations in haemagglutinin (HA), neuraminidase (NA), non-structural protein 1 (NS1), and polymerase basic 1 gene (PB1) [Reference Kawaoka and Webster33–Reference Miotto38]. However, a UK study that investigated the association between mutations in Flu Apdm09 and disease outcome [Reference Ellis39] observed that similar mutations (39/40) occurred in fatal and none-fatal cases suggesting that no significant virulence-related mutations occurred in influenza A(H1N1)pdm09 during the study period. It is thus unlikely that the severity observed in this study was due to mutations in influenza A virus. However, as mutations in RSV, RV, AdV, hMPV, and hPIV1–3 are largely not studied, the importance of these on virulence should be borne in mind when interpreting the results.
In summary, in this study, co-infection was associated with an increased risk of admission to a general ward, whereas single RSV or hPIV1–3 infections were associated with an increased risk of admission to a general ward, ICU or death. Evidence on the severity of disease by laboratory-confirmed respiratory virus infections is often lacking, the findings of this study offer a better understanding of the importance of respiratory viruses and help define a place for vaccine and drug development, especially vaccines and drugs which may target several of these viruses. This study also elucidates the importance of research on development of multi-target diagnostic tests.
ACKNOWLEDGEMENTS
The authors acknowledge the University of Manchester, the Manchester Academic Health Science Centre, and the Central Manchester University Hospitals NHS Foundation Trust and staff for their support in this research. This work received financial support from the University of Manchester.
DECLARATION OF INTEREST
None.








