INTRODUCTION
The Netherlands is a low-endemic country for hepatitis B: 2·1% of the population is anti-HBc positive and 0·2% HBsAg positive. All pregnant women are screened to prevent vertical transmission but no universal vaccination strategy is employed. In certain risk groups hepatitis B is more prevalent and poses a health problem. Therefore, the Dutch Ministry of Health pursues a vaccination policy for identified high-risk groups. One of the aspects of this risk-group policy is the national campaign directed at behavioural risk groups for hepatitis B, which started in November 2002 [Reference Heijnen1].
The campaign offers hepatitis B vaccination free of charge and is easily accessible to the risk groups. It aims at increasing the protection of hepatitis B within these groups and lowering HBV transmission. Target groups of the campaign are men who have sex with men (MSM), commercial sex workers (CSW) and their customers, current and former injecting and non-injecting hard-drug users (DU) and heterosexuals with a high rate of partner change (HS). Nationally coordinated by the Netherlands Association for Community Health Services, the 33 local Community Health Services (CHS) are responsible for the regional coordination and implementation of the campaign. The CHS cooperate with organizations such as addiction care organizations, gay bars and saunas, gay people's associations, sex clubs, red-light districts, STI outpatients' clinics, and prisons to reach the people at risk. The national registration is based on an internet application.
The campaign includes vaccination and primary screening of participants by testing for anti-HBcore during the first visit. A positive anti-HBcore test is interpreted as evidence of a hepatitis B infection in the past. Persons presenting with a positive anti-HBcore test undergo further testing for HBs antigen (HBsAg), to demonstrate an active HBV infection, with subsequent notification to the CHS. If no HBsAg is detected, immunity is assumed and no further vaccination is offered. Only persons with a negative anti-HBcore test are considered susceptible for infection and are offered further vaccination (three vaccinations at months 0, 1, 6). In this vaccination programme, which targets population groups and not individuals, the definite serological marker that proves protection (anti-HBs) is not considered necessary (Fig. 1).
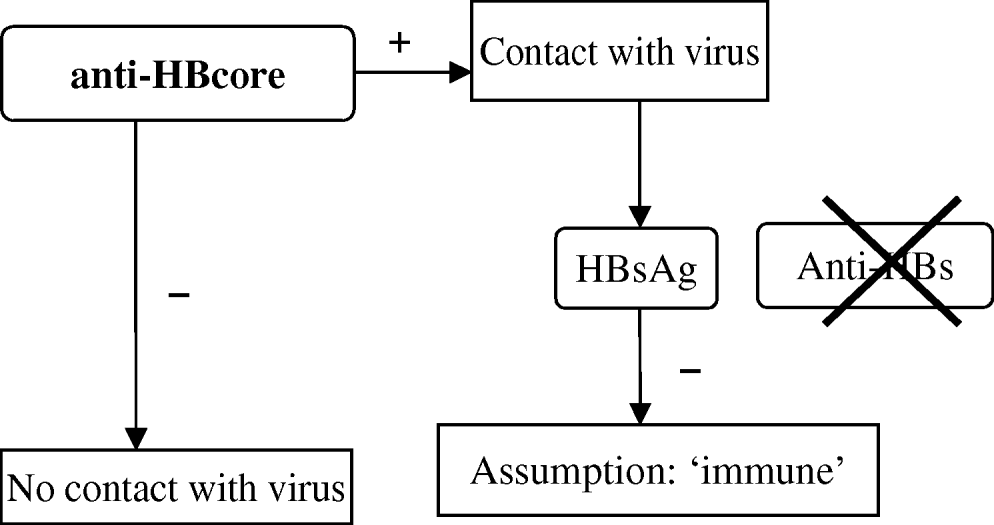
Fig. 1. Test algorithm in the Dutch HBV vaccination campaign.
However, it is known that anti-HBcore might occur without anti-HBs and in these cases immunity cannot be assumed proven (labelled as ‘isolated anticore’) [Reference Draelos2–Reference McMahon8]. A few individual observations during the campaign raised suspicion about the reliability of the assumption of immunity in the algorithm. One documented anti-HBcore-positive and HBsAg-negative case was subsequently not offered vaccination and developed acute hepatitis B infection afterwards. Some CHS noticed that DU who were routinely tested for anti-HBs frequently lacked this antibody.
Studies on the occurrence of isolated anti-HBcore showed a higher prevalence upon co-infection with HIV and HCV, suggesting suppression of anti-HBs production in anti-HBc-positive patients [Reference Gandhi3, Reference Wedemeyer9–Reference Helmy and Al-Sebayel11]. An isolated HBcore can be the result of a documented infection in the past without any demonstrable anti-HBs left (‘remnant antibody’), but could also result from a non-specific reaction to this test. In addition, low-level chronic infections, which lack both HBsAg and anti-HBs, occur and can give rise to an isolated anti-HBcore status [Reference Jilg4, Reference Jilg12–Reference Gross14]. Therefore, in an unknown number of participants of the Dutch hepatitis B vaccination campaign, the positive anti-HBcore could be the only positive (isolated) finding. These participants could be immune, but could also be experiencing an acute infection, be chronically infected or have a false-positive anti-HBcore.
The widespread practice of using this anti-HBcore assay as the defining test in algorithms on hepatitis B vaccination in risks groups gives rise to uncertainty which is derived from uncertainty concerning the performance of this assay with respect to sensitivity, specificity, reproducibility and lack of a recognized working standard for anti-HBc. We decided to evaluate, in a representative selection of participants in the campaign, the proportion of cases with actual evidence of immunity based on previous infection. It was intended to determine whether a relevant proportion lacked confirmation of immunity, in which case their exclusion from vaccination could have been unjustified. The results of this study could lead to a reconsideration of the test algorithm.
METHODS
Study design and population
In this cross-sectional study, 1000 blood samples from participants of the HBV vaccination campaign for behavioural risk groups were selected that already had been analysed as anti-HBcore positive and HBsAg negative. These were re-analysed in a central laboratory which had not participated in the initial testing. The samples were equally selected from the four risk groups: DU, HS, CSW and MSM.
Data collection
Three CHS linked to four laboratories participated at this study. The Netherlands Association for Community Health Services selected 1000 anti-HBcore-positive and HBsAg-negative participants from the central database with unique person identifiers, aiming at an equal distribution of the risk groups DU, HS, CSW and MSM, which are also documented in the database. The participating CHS supplied the corresponding identifying data to the laboratories that sent the available sera to the central laboratory. The specimens were kept in standard diagnostic laboratories, in which sera are stored at least at −20°C. The CHS provided the study coordinator with the corresponding demographic data.
Test algorithm
Isolated anti-HBcore is defined as a positive anti-HBcore test with negative HBsAg and negative anti-HBs. Possible causes of an isolated anti-HBcore are: (1) a past infection with loss of anti-HBs over time; (2) a chronic infection without demonstrable HBsAg, in which case HBV DNA is positive; (3) the core window phase of an acute hepatitis B infection, when HBs antibodies are not yet demonstrable; (4) false-positive anti-HBcore, sometimes associated with the presence of antinuclear antibodies and with the absence of anti-HBe and HBV DNA.
In order to determine the specificity of the positive anti-HBcore (HBsAg-negative) result additional tests were performed. First, the anti-HBcore was confirmed in a different laboratory. Subsequently, confirmed anti-HBc positive sera were tested for anti-HBs. Anti-HBs was considered negative if <4 IU/ml, according to the test manufacturer's instructions. The next step was to detect additional markers of HBV infection in anti-HBs-negative sera (the so-called ‘isolated’ anti-HBcore sera): anti-HBe and HBV DNA.
To evaluate the role of the reactivity (strength) of the anti-HBcore-positive findings we analysed the anti-HBs frequencies with different cut-off values of the anti-HBcore test. HCV tests were performed for DU only. HIV infection was assessed when enough serum volume was available.
Laboratory methods
Anti-HBcore, anti-HBs, anti-HCV and anti-HIV were determined using the AxSYM analyser (Abbott Diagnostics, Wiesbaden, Germany). Anti-HBe was determined using the Biokit EIA (Biokit S.A., Barcelona, Spain). HBV DNA was determined for confirmation of a positive anti-HBc by real-time PCR, targeting the preS1/preS2 region s gene, detection by molecular beacon, with phocine herpesvirus gB gen as internal control on a Bio-Rad iCycler system (Bio-Rad Laboratories, Veenendaal, The Netherlands). Primers: sense 5′-GGCGTTTTATCATMTTCCTCT-3′; antisense: 5′-CAACATACCTTGRTAGTCCAGAA-3′ and Taqman probe: 5′-CCTGCTGCTATGCCTCATCTTCT-3′ were used. Fragment size: 80 bp.
Statistical analysis
Data were statistically analysed using SPSS version 12.0 (SPSS Inc., Chicago, IL, USA). Differences in proportions were tested using χ2 tests. A P value of <0·05 was considered significant, and 95% confidence intervals (CI) were calculated. Univariate logistic regression analyses were performed, with age, sex, risk group, HCV infection in drug users, HIV infection, and being born in a HBV-endemic country as independent variables and isolated anti-HBc as the dependent variable. For the odds ratios, 95% CI were calculated. Variables showing an association of P<0·2 were included in the multivariate analysis. HCV could not be included in the model as a separate variable as it was only available for the risk group DU. In order to include HCV in the multivariate analyses, a new variable for the target group was created which included three levels for the DU according to their HCV status. Backward stepwise selection was performed with a P value for the likelihood ratio test >0·10 as the criterion for the elimination of variables from the model.
RESULTS
A total of anti-HBcore-positive/HBsA-negative sera of 1013 participants were collected, almost equally divided among the four risk groups. CHS no. 1 provided 72·5% (734/1013) of the samples, CHS no. 2 provided 21·3% (216/1013) and CHS no.3 provided 6·2% (63/1013). The mean age of the participants was 39 years (range 16–74 years) and 33·2% (336/1013) were female.
The participants originated from 88 different countries. Of all the participants, 18·1% (177/975) originated from countries with high endemicity for hepatitis B (prevalence of HBsAg 8% of the above), in comparison to 35·3% (344/975) with intermediate endemicity (prevalence between 2% and 8%) and 46·6% (454/975) with low endemicity (prevalence <2%). Of the 454 participants from low-endemic countries, 342 were of Dutch origin. HIV infection was found in 10·1% (88/874) of the participants (Table 1).
Table 1. Demographic and serological characteristics of participants in the HBV vaccination campaign in The Netherlands (2003–2005)
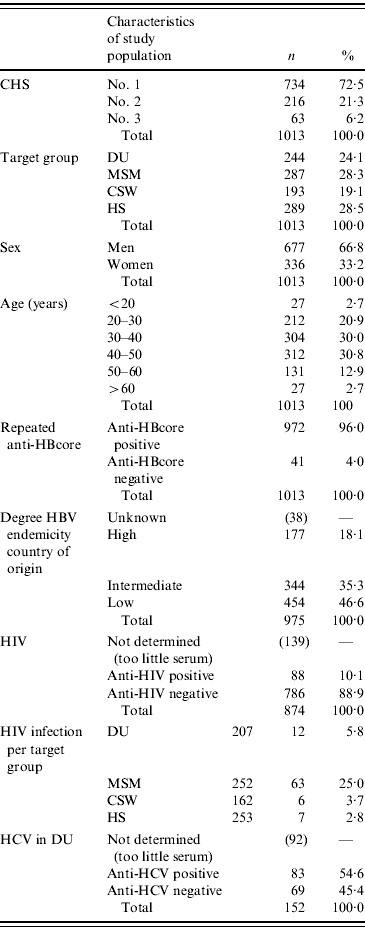
DU, Hard-drug users; MSM, men who have sex with men; CSW, commercial sex workers; HS, heterosexuals with a high rate of partner change.
After retesting, the initial anti-HBcore-positive test could not be confirmed in 4·0% (41/1013) of the sera (range between laboratories 3–18%). We tested 12/41 HBe antibodies in these unreproducible anti-HBcore-positive samples. They all proved anti-HBe negative.
Of the 972 sera with confirmed positive anti-HBcore, 141 (14·7%, 95% CI 12·6–17·1) were anti-HBs negative, the so-called isolated anti-HBcore (Fig. 2). Using various cut-offs for the anti-HBcore did not significantly influence the percentage of isolated anti-HBcore. Markedly, 7/40 (17·5%) of those sera in which the anti-HBcore could not be confirmed were anti-HBs positive (Table 2).
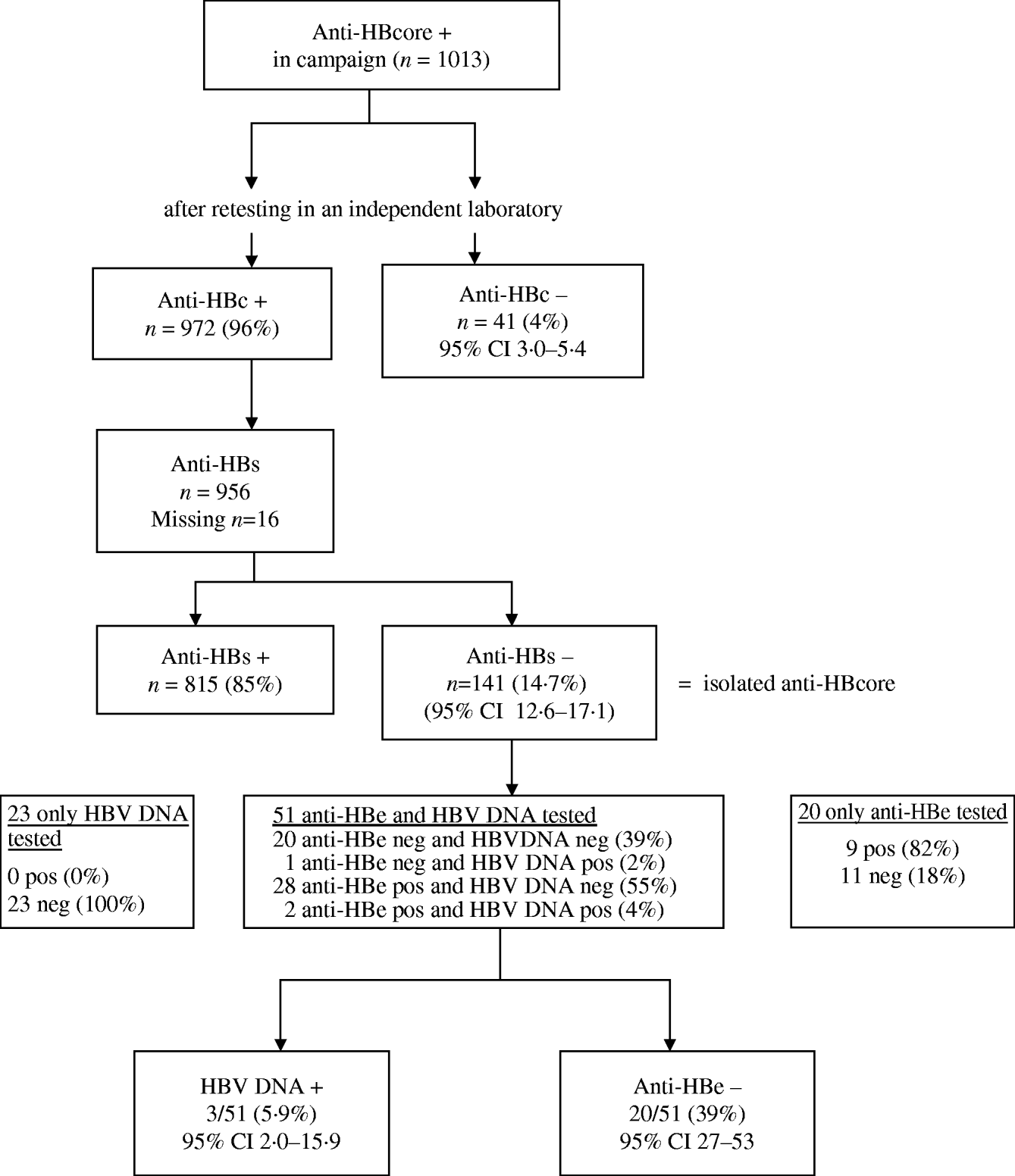
Fig. 2. Test algorithm and results of laboratory testing.
Table 2. Percentage anti-HBs related to various anti-HBcore cut-off values
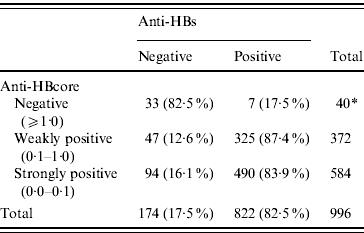
* In one anti-HBcore-negative sample anti-HBs was not determined.
Isolated anti-HBcore was not associated with age and sex. The prevalence of isolated anti-HBcore among DU was significantly higher compared to other risk groups: 22·4% (51/228). Among MSM the prevalence was lowest (6·3%); among CSW and HS it was 15·8% and 16·1% respectively. For one third of DU, insufficient serum remained for HCV testing. Of DU who could be tested for HCV, the percentage of isolated anti-HBcore in those who were anti-HCV positive was significantly higher (25·3%, 21/83) than in those anti-HCV negative (13·0%, 9/69; P=0·059). The prevalence of isolated anti-HBc was significantly higher among people from hepatitis B high-endemic countries (23·0%, 38/165), compared to intermediate and low-endemic countries (13·4%, 101/753; P<0·002). Within hepatitis B high-endemic countries, the prevalence was significantly higher among participants from Sub-Saharan African countries (27·1%, 32/118), compared to other high-endemic regions (12·8%, 6/47; P<0·035).
In multivariate analysis, target groups including HCV for DU and HBV endemicity in the country of origin remained independent predictors of isolated anti-HBcore. Compared to the target group HS, MSM had a lower rate of isolated anti-HBcore (OR 0·5, 95% CI 0·2–0·9), while HCV-positive DU had a higher rate (OR 2·5, 95% CI 1·3–4·7). Participants born in Sub-Saharan Africa had a higher rate of isolated anti-HBcore (OR 2·6, 95% CI 1·6–4·4), compared to low- and intermediate-endemic countries, and also compared to other high-endemic countries (Table 3).
Table 3. Determinants isolated in anti-HBcore after test on anti-HBs
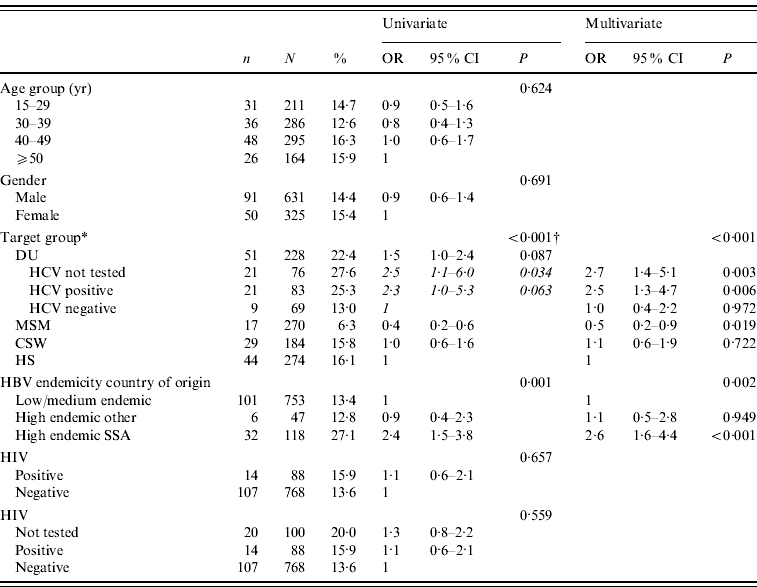
* DU, Hard-drug users; MSM, men who have sex with men; CSW, commercial sex workers; HS, heterosexuals with a high rate of partner change; SSA, Sub-Saharan Africa.
† P value of univariate logistic regression of main target groups, the results of univariate logistic regression of the DU subgroups are shown in italics.
Because of insufficient serum, we could not determine anti-HBe and HBV DNA in all sera. Of the 20/51 sera tested on both anti-HBe and HBV DNA, 39% (95% CI 27–53%) proved to have no indicator for infection. Three of these 51 sera (5·9%, 95% CI 2·0–15·9) were HBV DNA positive (range 40–6600 virus copies/ml) (Fig. 2).
On the basis of these results, an estimate was made of the proportion of unconfirmed and isolated anti-HBcore, after extrapolating to the total population during the 4 years of the campaign. For this purpose, anti-HBe-positive participants were considered immune. On the basis of this calculation, in 9·6% of the persons in which immunity was assumed, there was no additional evidence available to support this. This proportion varied between risk groups; from 6·5% in MSM to 12·5% in DU (Table 4).
Table 4. Prevalence of anti-HBs, anti-HBe and HBV DNA in participants of the HBV campaign in The Netherlands, after extrapolation
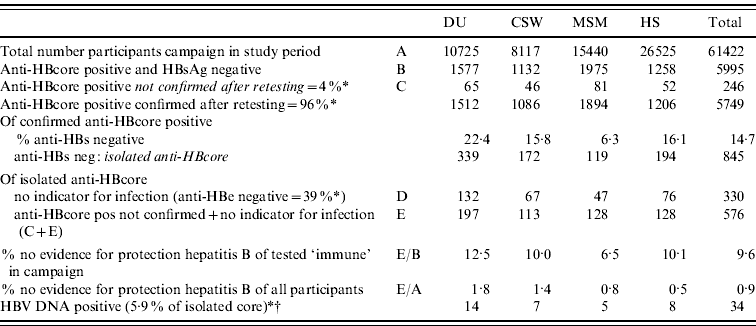
DU, Hard-drug users; CSW, commercial sex workers; MSM, men who have sex with men; HS, heterosexuals with a high rate of partner change.
* Percentages based on results of this study.
† No specific percentage per target group because of low numbers (5·9%=3/51).
The results of the HBV DNA analysis were not included in this calculation because, while HBV DNA-positive persons did receive an incorrect diagnosis (not protected but infected with a low HBV viral load), they were not unjustly excluded from vaccination.
DISCUSSION
Targeting hepatitis B vaccination to high-risk groups, which is a common practice in countries of low endemicity including The Netherlands, directly implies the frequent occurrence of pre-existing immunity among participants, because a high-risk group has by definition had an increased risk for exposure. To avoid unnecessary vaccination, pre-vaccination screening is often employed and for this purpose the anti-HBcore antibody test is widely used. This study evaluated the use of anti-HBcore testing by focusing on the reliability of the assumption of immunity in cases that tested positive in a public health-based hepatitis B vaccination programme for behavioural high-risk groups. To our knowledge, no previous reports on the significance of isolated anti-HBcore in this setting are available. Our study shows a frequent isolated anti-HBcore in this population of 14·7%, and even higher in the subgroups of hard-drug users and immigrants. Persons originating from countries of high endemicity and HCV-positive DU showed significantly higher proportions with this finding.
The first problem we encountered was that, after retesting the anti-HBcore-positive samples, the initial positive result could not be confirmed in 4% of the cases. The irreproducible result of the first test could mean this first result was false positive. This is confirmed by the finding of 12 retested specimens which proved to be anti-HBe negative. It is remarkable that regarding this diagnostic test for antibodies to the core antigen of HBV, no international standard or working standard is available for calibration or reference purposes. Neither in the literature, nor in the package insert of commercial assays, is validation described for the anti-HBcore test by means of an accepted standard. This concern has not precluded certification of this established test, probably as this test is not considered to play a major role in the primary diagnosis of HBV infections. Inevitably, due to this circumstance, non-specific or false-positive results are difficult to recognize. In one laboratory the percentage of positive anti-HBcore that could not be reproduced after retesting was markedly high (18%), which could be attributed to technical differences between manufacturers, underlining the problem of the lack of an established standard.
We did not observe a higher prevalence of isolated anti-HBcore among the ‘weaker’ (cut-off values 1·0–0·1) positive anti-HBcore results. Choosing a low cut-off (<0·1) did not contribute to an improved specificity, in terms of more frequent occurrence of anti-HBs. In contrast to our study, an earlier German study among blood donors did find that high test values correlated with results that could more often be confirmed by additional tests [Reference Schmidt15]. The finding of anti-HBs in a small proportion of the anti-HBcore-negative individuals could be considered significant. It seems likely that this finding is related to confusion with regard to vaccination status in the established risk groups. One should also consider the theoretical possibility that the anti-HBcore test is false negative under some circumstances, again because there is no definite proof of the status of this test.
The occurrence of isolated anti-HBcore in this study was 14·7%, with considerable variation between risk groups. There is limited data on the prevalence of isolated anti-HBcore in various risk groups. In a French study among 391 prisoners (23% of whom had never used intravenous drugs) 124 had a positive anti-HBc. Of those, five were infected (HBsAg positive) and 21% (25/119) had an isolated anti-HBcore [Reference Rotily16]. The isolated anti-HBcore prevalence in risk groups in hepatitis B low-endemic countries varies from 0·3 to 1·1%, on the basis of older studies in blood donors [Reference Schifman17, Reference Schmidt, Leparc and Samia18]. A total of 18–54% of anti-HBcore-positive blood donors had an isolated anti-HBcore. These data give limited information about the prevalence of isolated anti-HBcore among groups at risk because of their behaviour.
The prevalence of isolated anti-HBcore in anti-HBc-positive participants in this study was highest in DU (22·4%), which rose to 25·3% if anti-HCV was positive. This confirms the previously described finding that isolated anti-HBcore is significantly more prevalent among DU co-infected with hepatitis C [Reference Gandhi10, Reference Rodriguez-Mendez19]. This could be the result of a false- positive anti-HBcore because of theoretical cross-reactivity with anti-HCV antibodies or the loss of anti-HBs in a past infection [Reference Wedemeyer9, Reference Helmy and Al-Sebayel11]. Especially among DU, the outcome of the test offers little certainty of protection. The prevalence of isolated anti-HBcore in DU who could not be tested for HCV (28%) was similar to that in HCV-positive DU (25%). This may be an indication that most of those were also HCV infected.
In MSM the frequency of isolated anti-HBcore was lower than in the other target groups. One possible explanation is the occurrence of recently acquired HBV infections in MSM, where anti-HBs is still measurable. Contrary to reports in the literature [Reference Rodriguez-Mendez19] we did not find significantly more anti-HBcore in HIV-infected people.
Isolated anti-HBcore was statistically more prevalent among people from hepatitis B high-endemic countries compared to medium- and low-endemic countries, especially in participants from Sub-Saharan African countries compared to other high-endemic countries. This could either be related to infections long ago, with a single remnant antibody left, or to the more frequent occurrence of non-specific results in persons originating from areas with high infection rates in general.
The present study suffered to some extent from limitations arising from the available serum volumes, which hampered the implementation of the intended additional testing. Anti-HBe and HBV DNA was determined in only half of the available confirmed anti-HBcore-positive sera. However, we believe that these additional results do allow some conclusions after extrapolation.
In 3/51 (5·9%) anti-HBs-negative samples we found low levels of HBV DNA. A low prevalence of DNA-positive samples in isolated anti-HBcore has been found in other studies [Reference Jilg4, Reference Helmy and Al-Sebayel11, Reference Weber20]. In those cases, however, the people would not have benefited from vaccination. This type of occult hepatitis B infection is not considered infectious in social contacts. Still, these persons had been incorrectly labelled as being protected from infection. A total of 61% of the anti-HBs-negative samples turned out to be anti-HBe positive, which indicates that over half of the participants with an isolated anti-HBcore experienced a previous infection. In the remaining cases, protection by previous infection has to be considered unproven. Overall for 9·6% (almost 12·5% for DU) of the participants in the campaign who had been told that they were protected on the basis of the result of the blood test, this result could not be confirmed. This indicates the maximum proportion of persons who were unjustly excluded from further vaccination.
In summary, this study showed that isolated anti-HBcore occurs in a small but significant proportion of participants in high-risk populations. In these persons, immunity cannot be considered proven and low-level chronic infections may even be present. We conclude that the screening by anti-HBcore alone, is not reliable, if considered from the viewpoint of individual care. We emphasize that, if a test result remains unconfirmed, the benefit of the doubt should primarily go to the individual at risk, from whom vaccination would otherwise be withheld. It is therefore recommended that anti-HBs testing is included in pre-vaccination screening algorithms, either together with HBsAg or after initial positive anti-HBcore result, to provide maximum confidence in the assumed protection in individual cases. Immunity that cannot be confirmed by any practical means leads to the necessity of completing the vaccination series, to avoid any risk of sustained susceptibility in individuals at high risk of infection.
ACKNOWLEDGEMENTS
We thank Irene Veldhuijzen (Municipal PublicHealth Service Rotterdam Rijnmond) and Hans Bor (Radboud University Nijmegen Medical Centre) for their much appreciated contribution to the statistical analysis of the results of the laboratory testing. We also thank the regional microbiological laboratories of Amsterdam, Rotterdam and Nijmegen for making their sera available, and that of Leiden for processing the samples. Thanks are due to Yvonne Montfoort for improving and correcting language issues.
DECLARATION OF INTEREST
None.








