INTRODUCTION
Helicobacter pylori infection is one of the most common chronic bacterial infections in humans. The infection occurs worldwide and clinical symptoms develop only in 10–20% of infected people [Reference Suerbaum and Michetti1]. H. pylori causes gastritis, gastric and duodenal ulcers [Reference Suerbaum and Michetti1–Reference Verdu3], and it increases the risk of gastric carcinoma and gastric mucosa-associated lymphoid tissue lymphoma [Reference Suerbaum and Michetti1–Reference Verdu3]. The prevalence of H. pylori infection is ⩾80% in adults from developing countries compared to 20–50% in developed countries [Reference Suerbaum and Michetti1, Reference De Vries4]. In industrialized countries, a high prevalence of the infection is found in immigrants from highly endemic countries [Reference Rothenbacher2–Reference De Vries4]. Low socioeconomic status, living in crowded conditions in childhood, and family members infected with H. pylori are the main risk factors for the infection [Reference Bani-Hani5–Reference Weyermann, Rothenbacher and Brenner7]. The prevalence of H. pylori infection has decreased in the past decades in developed countries [Reference Suerbaum and Michetti1, Reference Frenck and Clemens8]; explanations for this trend include improvements in sanitation and living conditions [Reference Tkachenko9–Reference Ozden10], decreasing family size [Reference Cover and Blaser11] and antibiotic use [Reference Rothenbacher, Bode and Brenner12–Reference Broussard13].
The prevalence of H. pylori infection has been studied previously in Israel in small and selected samples. The prevalence in adults aged ⩾30 years residing in rural areas was 72% [Reference Gilboa14], 50% in Israeli Arab children aged 3–5 years [Reference Muhsen15], 60% at age 6–9 years [Reference Muhsen16], and 25% in Jewish children aged 3–60 months [Reference Kori, Goldstein and Granot17], suggesting ethnic differences in H. pylori infection prevalence in Israel. The Israeli population is a mixture of population groups originating from almost all countries around the world. About one third (29%) of the Jewish population in Israel in 2009 was born abroad. The present study provided a rare opportunity to examine the combined impact of population ethnic and origin diversity, as well as socioeconomic status on the prevalence of H. pylori infection.
The aims of the study were to examine the prevalence, correlates and trends of H. pylori infection in the Israeli population, in two large national samples obtained 7 years apart: 2000–2001 and 2007–2008. The main study hypothesis was that H. pylori infection prevalence will be age- and ethnicity-dependent and there will be a decrease in the prevalence rate between the two time periods.
METHODS
Study population
The study was conducted in the Israeli population, which is comprised of two major ethnic groups: the Jewish population (80%) and the Israeli Arab population (20%).
The growth of the Jewish population results from both natural increase (births) and massive immigration of Jews from all over the world to Israel. Among Israeli Arabs, however, natural growth is the only source of population increase.
Sampling and collection of data and sera
Stored sera that were collected in 2007–2008 in the framework of the serum bank of the Israel Center for Disease Control were used. To examine changes in H. pylori seroprevalence over time, sera obtained from children and adolescents in 2000–2001 by the same method and sampling framework were also tested for the presence of anti-H. pylori IgG antibodies and compared to the 2007–2008 survey. The serum bank comprises residual sera obtained from diagnostic laboratories for subjects aged <18 years, and from healthy adult blood donors (⩾18 years). The samples were collected from all regions in Israel, from both males and females of all ages. Collection of sera is continuous throughout the year. Both sources exclude duplicate samples from the same individual, as well as sera taken from subjects with confirmed or suspected immunological disorders. Sera are kept frozen at −70°C until required.
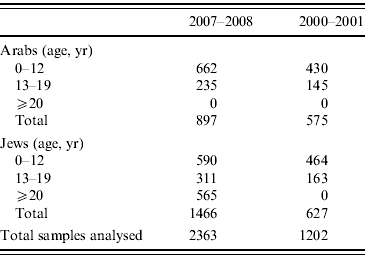
The samples are coded with a unique identifier and the following variables are known for each sample: sex, age, place of residence (at the level of town), population group (Jewish or Arab), and country of birth of the individual and his/her father. Family origin for the Jewish participants was determined by the father's country of birth. This variable was grouped as follows: Israel, North America/Western Europe/Australia, Asia/Africa/South America, or Russia/Eastern Europe. Serum samples from the Arab population were collected mainly from children and adolescents, since this population is not well represented among blood donors (the source of the adult samples). Overall 2556 samples in the 2007–2008 survey and 1206 samples in the 2000–2001 survey were randomly selected from the serum bank using an age- and sex-stratified sampling design. Of these 2363 and 1202 samples from the 2007–2008 and 2000–2001 survey, respectively, were included in the analysis (Table 1).
Table 1. Number of serum samples used for the analysis by collection period and selected parameters
The socioeconomic rank of the town of residence as ranked by the Central Bureau of Statistics [18] was used as a marker for socioeconomic status (SES). The ranks are on a scale of 1–10, the lower the rank, the lower is the SES. This SES index reflects a combination of basic factors, e.g. financial resources, household crowding, motorization, educational level, employment, etc. [18]. The SES of Jewish subjects living in towns at socioeconomic ranks 1–4 was considered as low SES, 5–6 as intermediate SES, and ⩾7 as high SES. The SES of Arabs living in towns at rank 1 was classified as low SES, while ranks 2–3, and ⩾4 were classified as intermediate and high SES, respectively.
Detection of anti-Helicobacter pylori IgG antibodies
H. pylori-specific IgG antibodies were measured in all the serum samples using Dade Behring's Enzygnost® Anti-Helicobacter pylori II/IgG commercial kit (Dade Behring, Germany) according to the manufacturer's instructions. The sensitivity and specificity of the kit are 93·4% and 98·8%, respectively. For children, the sensitivity and specificity of this kit are 92·7% and 95·7%, respectively, and in children aged <6 years the sensitivity and specificity are 91·6%, respectively [Reference Kindermann19]. Sera with results of ⩾0·250 IU were considered positive for H. pylori IgG antibodies.
Statistical analysis
Seroprevalence of H. pylori IgG antibodies was described by population group: Jews or Arabs. Direct adjustment was applied to calculate the age-adjusted prevalence [and 95% confidence intervals (CIs)] while using the Israeli population in 2008 as the reference population, and to calculate the SES-adjusted prevalence (and 95% CIs), while using the overall 2007–2008 sample as the reference population. SES-adjusted prevalence was calculated to control for SES differences in participants from the 2000–2001 and 2007–2008 surveys. Age-specific prevalence (and 95% CIs) was calculated. Differences in the prevalence of H. pylori infection were examined by sex, age groups, family origin (only for the Jewish population), and community SES using χ2 test, and multivariate analyses applying generalized linear models. Crude and adjusted prevalence ratio (PR) and 95% CIs were calculated. ANOVA was used to examine the difference in the mean age according to family origin, and Bonferroni's test was used to correct for multiple comparisons. Two-tailed P<0·05 was considered statistically significant. Data were analysed using SPSS version 17 (SPSS Inc., USA) and WINPEPI software [Reference Abramson20].
RESULTS
Among 1466 Jewish participants of the 2007–2008 survey there were 762 (52·0%) males and 704 (48·0%) females; the mean age was 20·2 (s.d.=16·7) years (range 0–77 years). From the same survey 897 Arab participants were included in the analysis; 484 (54·0%) were males and 413 (46·0%) were females; their mean age was 8·9 (s.d. =5·1) years (range 0–19 years).
Jewish participants
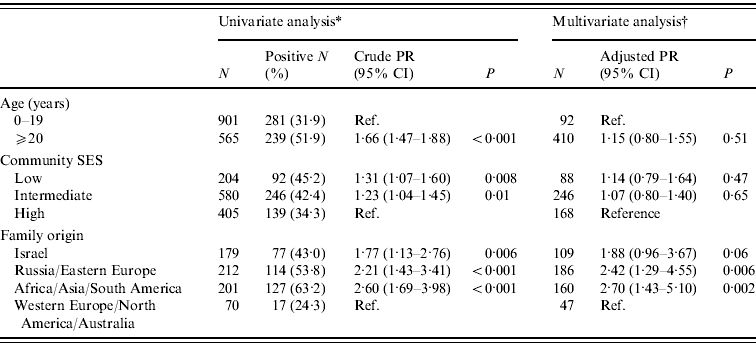
In the 2007–2008 survey, there were 574 (39·2%) samples that tested positive for H. pylori. The age-adjusted prevalence proportion was 45·2%. H. pylori seropositivity increased significantly with age (χ2 for trend 121·27, P<0·001). It was 13·3% in children aged <5 years and increased sharply by more than twofold at age 5–8 years. Among older children, a continuous but modest increase in H. pylori prevalence was observed. In adolescents and young adults, the prevalence remained stable (42–46%), and it reached 60% in the ⩾50 years age group (Fig. 1). No significant difference was found between males and females in H. pylori seropositivity: 40·2% and 38·1%, respectively (P=0·41). H. pylori prevalence increased with decreasing SES (χ2 for trend 8·18, P=0·004) (Table 2). Data on family origin were available for 662 subjects (45·2% of the Jewish participants). The prevalence of H. pylori infection varied significantly by family origin (P<0·001), being lowest (24·3%) in subjects originating from North America/Western Europe/Australia, and highest (63·2%) in subjects originating from Asia/Africa/South America (Table 2).

Fig. 1. Helicobacter pylori IgG antibody seroprevalence in Arabs (□) and in Jews (
![]() ) in Israel, 2007–2008.
) in Israel, 2007–2008.
Table 2. Univariate and multivariate analyses of H. pylori IgG seroprevalence correlates in the Jewish population, Israel 2007–2008
SES, Socioeconomic status.
* Crude prevalence ratio (PR) and 95% confidence intervals (CI). Significance level of the univariate analysis was determined by χ2 test.
† Adjusted prevalence ratio, 95% confidence intervals and significance levels were obtained through multivariate analysis using generalized linear models. The analysis adjusted for the variable in the tables and for sex.
Family origin was the only significant correlate of H. pylori infection in a multivariate analysis that adjusted for the variables: age, sex and community SES. Second-generation subjects in Israel and those whose family origin was Asia/Africa/South America, and Russia/Eastern Europe had a 1·88–2·70 greater risk of H. pylori infection than subjects whose family origin was North America/Western Europe/Australia (Table 2). The variables age and family origin were significantly associated; second-generation born in Israel were younger (mean age 25·6, s.d.=7·7 years), than the other groups; the mean (s.d.) ages were 37·3 (14·7) years in subjects originating from Asia/Africa/South America, 36·5 (16·0) years in subjects originating from Russia/Eastern Europe, and 32·7 (15·8) years, in those originating from North America/Western Europe/Australia (P<0·001 for the difference in groups). Given the significant associations between age and H. pylori seropositivity, and between family origin and age, the analysis was repeated while excluding subjects who were second-generation born in Israel. In this model that included 393 participants, subjects originating from Asia/Africa/South America had 46% increased prevalence of the infection (adjusted PR 1·46, 95% CI 1·24–1·72, P<0·001), and those originating from Russia/Eastern Europe had 38% increased risk of the infection (adjusted PR 1·38, 95% CI 1·18–1·62, P<0·001), compared to those originating from North America/Western Europe/Australia. Participants aged ⩾20 years had 14% higher prevalence of the infection compared to younger participants (adjusted PR 1·14, 95% CI 1·00–1·29, P=0·03).
Arab participants
Serum H. pylori IgG antibodies were detected in 377 (42·1%) participants of the 2007–2008 survey. H. pylori seropositivity increased substantially and significantly with age (χ2 for trend 101·27, P<0·001), from 22% in children aged ⩽4 years, to 60–65% in the 13–19 years age group (Fig. 1). The prevalence of H. pylori infection was consistently higher in Arab children and adolescents than their Jewish peers (Fig. 1). There was no significant difference in H. pylori seroprevalence by sex: 40·3% in boys and 44·6% in girls (P=0·19). H. pylori seroprevalence was 33·0% in subjects from high SES communities compared to 36·2% and 58·7% in subjects from intermediate and low SES communities, respectively (χ2 for trend 33·32, P<0·001). Age and community SES retained statistically significant associations with H. pylori infection in multivariate analysis (Table 3).
Table 3. Univariate and multivariate analyses of H. pylori IgG seroprevalence correlates in Arab participants, Israel, 2007–2008
SES, Socioeconomic status.
* Crude prevalence ratio (PR) and 95% confidence intervals (CI). The significance level of the univariate analysis was determined by χ2 test.
† Adjusted prevalence ratio, 95% confidence intervals and significance levels were obtained through multivariate analysis using generalized linear models. The analysis adjusted for the variable in the tables and for sex.
Trends in H. pylori seroprevalence between 2000–2001 and 2007–2008
The age-adjusted H. pylori seroprevalence in Jewish participants aged 0–19 years in 2000–2001 was 23·4% (95% CI 20·1–26·8), and 31·2% (95% CI 28·2–34·1) in 2007–2008. The respective SES-adjusted H. pylori seropositivity was 27·3% (95% CI 23·2–31·5) and 32·9% (95% CI 29·5–36·2). The age-adjusted H. pylori seropositivity in Arabs in 2000–2001 was 48·2% (95% CI 44·2–52·1), and 42·8% (95% CI 39·7–45·9) in 2007–2008, and the respective SES-adjusted seroprevalence was 44·6% (95% 40·1–49·0), and 40·5% (95% CI 37·3–43·7), indicating no significant difference between the two surveys in either ethnic group.
DISCUSSION
Our study provides data on the seroprevalence of H. pylori infection in relation to ethnicity and population migration. The current study also provides information on the trends in H. pylori infection using sera that were obtained 7 years apart.
We used convenience samples of residual sera from diagnostic laboratories and blood donors. Using such convenience samples was shown to yield comparable estimates of immune-profiles of various vaccine-preventable diseases to those obtained through multi-stage cluster random samples [Reference Kelly21]. The sera samples in our study were representative of the various population groups in terms of sex, age and family origin (for the Jewish population). With regard to ethnicity, however, adults from the Israeli Arab population were not well represented.
The age-adjusted prevalence of H. pylori infection in the Jewish population was 45·2%. The prevalence of H. pylori infection increased significantly with age. H. pylori infection is acquired in childhood [Reference Malaty22–Reference Rowland24]; therefore, the increasing infection prevalence between preschool age, school age and adolescence possibly reflects the acquisition of the infection, while the increase between adolescence and adulthood possibly results from birth cohort effect. This pattern of rising H. pylori infection prevalence with age has been observed in previous studies [Reference Malaty22, 25–Reference Bakka and Salih28]. H. pylori seroprevalence in adults in our study ranged from 42% to 60%, and this is comparable with the prevalence found in some developed countries [Reference Palli27, Reference Rothenbacher and Brenner29, Reference Martin-de-Argila30]. However the prevalence of H. pylori infection in Israeli children and adolescents is higher than in children from developed countries [Reference Rothenbacher and Brenner29].
An inverse association was found between H. pylori infection and SES. This is in agreement with previous studies, in which markers of low SES such as low education, crowded households, and low family income, were associated with higher risk of H. pylori infection [Reference Bani-Hani5, Reference Malaty6, Reference Palli27, Reference Bakka and Salih28, Reference Opekun31]. Interestingly, the prevalence of H. pylori infection differed significantly according to family origin. It was highest (63·2%) in subjects originating from countries of high endemicity for H. pylori infection, i.e. Asian, African, and South American countries, followed by 53·8% in subjects whose family origin was Russia/Eastern Europe, Israel (43·0%), and North America/Western Europe/Australia (24·3%). Family origin was a significant and strong predictor of H. pylori infection in multivariate analyses after controlling for age and community SES. A previous study on H. pylori infection from Israel [Reference Gilboa14] reported a similar finding. It is therefore suggested that family origin reflects better the household living conditions in childhood, than current SES. Family members infected with H. pylori are the main reservoir and a major risk factor of H. pylori infection [Reference Weyermann, Rothenbacher and Brenner7, Reference Muhsen15, Reference Muhsen16, Reference Rothenbacher32–Reference Dominici34]. The intra-familial transmission of H. pylori infection might be long term and of sustained influence [Reference Muhsen16]. A greater prevalence of H. pylori infection is expected in people originating from countries with high endemicity for H. pylori infection. Therefore subjects born and raised in such families are assumed to be more often, and more intensively, exposed to the natural reservoir of H. pylori than those whose origin is from a developed country.
The prevalence of H. pylori infection in Arab participants was higher than their Jewish peers. Supported by our previous studies [Reference Muhsen15, Reference Muhsen16], the prevalence of H. pylori infection in this population is similar to that observed in children from developed countries [Reference Bani-Hani5, Reference Bakka and Salih28, Reference Mohammad35]. The Arab population in Israel has lower socioeconomic indicators and larger families compared to the Jewish population, and that might contribute to the differences in H. pylori seroprevalence between the two groups.
Differences in the prevalence of H. pylori infection in subpopulations have been described previously [Reference Rothenbacher2–Reference De Vries4, Reference Malaty22]. In the USA, for example, the prevalence of H. pylori infection was 28·4% in non-Hispanic White, 63·0% in Mexican American and 54·0% in non-Hispanic Black participants [Reference McQuillan36], these differences persisted after controlling for SES indicators [Reference McQuillan36]. In The Netherlands, the prevalence of infection was 46% in Dutch participants compared to 78% in subjects of immigrants of non-Dutch ethnicity [Reference De Vries4].
The prevalence of H. pylori infection has declined in the past decades [Reference Tkachenko9, Reference Ozden10, Reference Banatvala37–Reference Fujisawa40]. Our data showed no significant change in H. pylori infection prevalence between 2000–2001 and 2007–2008, when controlling for SES. The comparison of our findings with a previous study from Israel that reported a prevalence of 72% in adults, suggests a decline in H. pylori infection. Further evidence of such a decrease comes from our survey when comparing the prevalence of infection in adult cohorts; 55–60% in those aged ⩾30 years and 42% in the 20–29 years age group.
Our study provides updated national information on the epidemiology and trends of H. pylori infection in a large sample of the Israeli population. Including both ethnic groups living in Israel; the Arab and Jewish population, and various segments of the Jewish population originating from different continents, our findings can be relevant to other Western countries characterized by ethnic diversity and population migration. However, the study has several limitations. Using socioeconomic rank of place of residence might have induced non-differential misclassification of the individual-level SES. The generalizability of the prevalence estimates might be limited since the Arab population and ultraorthodox Jewish population are underrepresented in adults in the serum bank.
In conclusion, our findings indicate a relatively high prevalence of H. pylori infection in Israel, especially in Jews originating from countries of high endemicity for H. pylori infection, and in Arabs.
ACKNOWLEDGEMENTS
This study was in part supported by the Stanley Steyer Institute for Cancer Epidemiology and Research, Sackler Faculty of Medicine, Tel-Aviv University, Tel Aviv, Israel. The authors thank Ms. Ravit Bassal for help with data collection and Ms. Sophy Goren for help with data management.
DECLARATION OF INTEREST
None.






