INTRODUCTION
Toxoplasmosis is an infection that occurs worldwide, with higher incidence in tropical areas. On the other hand, incidence decreases in cold areas and high latitude. It is a common infection in Central and South America, especially in regions with a moderate climate [Reference Petersen1].
The infection is caused by Toxoplasma gondii, an obligate intracellular protozoan and may take several different forms: tachyzoites which rapidly multiply and destroy tissue during acute infection; bradyzoites which slowly multiply in body tissues, leading to dormant forms – cysts, and oocysts, which are excreted by recently infected cats in their faeces. It is one of the most evident members of the phylum with about a third of the human population being chronically infected [Reference Montoya and Liesenfeld2, Reference Montoya and Rosso3]. The parasite can be transmitted to the foetus by the passage of tachyzoites through the placenta [Reference Petersen1].
The age-specific prevalence has been decreasing in some European countries over the past three to four decades [Reference Petersen1], but Ades & Nokes pointed out that in England stability has been observed since the 1970s after a sixfold decay in prevalence prior to that time [Reference Ades and Nokes4]. This is a demonstration that toxoplasmosis varies from country to country, and even from time to time in the same country.
Many studies have shown that 50–80% of Brazilian women that are pregnant or of childbearing age have antibodies against T. gondii [Reference Nóbrega and Karnikowski5]. Brazil is a very large country, with heterogeneous access to healthcare and with considerable demographical, socioeconomic and cultural differences, which may explain variations in diverse studies and localities.
A number of factors influence the level of exposure to T. gondii in the population, e.g. ingestion of raw or poorly cooked meat, intake of contaminated vegetables, close association with cats [Reference Avelino6], ingestion of non-filtered water, rural residential location [Reference Bahia-Oliveira7] and contact with contaminated ground [Reference Spalding8].
Toxoplasmosis has a significant impact from the public health perspective, mainly due to the risk of disease transmission through pregnancy [Reference Elsheikha9]. Congenital infection still occurs, despite the availability of diagnostic examinations and therapy worldwide. In most cases it is the result of primary infection of a pregnant woman [Reference Montoya and Liesenfeld2].
Vertical transmission of T. gondii may cause important morbidity and mortality to the foetus and the newborn, but in most cases the infants are asymptomatic at birth, developing the disease at any age [Reference Montoya and Rosso3]. The main frequent manifestations of congenital disease are learning and visual disabilities [Reference Jones10].
Worldwide, 3–8/1000 live births are infected in the uterus. There are many risk factors for the infection of the foetus, including the rate of transmission, mother's age, climate, area of residency, lifestyle, strain virulence, education and eating habits. Thus, this combination leads to marked differences even within countries [Reference Elsheikha9, Reference Rorman11].
In Guatemala the estimated incidence of congenital toxoplasmosis reported in a study carried out in newborns was 10·9/1000 live births, although the authors believe that the incidence found was lower than the expected because the region is known for a high incidence of this infection among pregnant women [Reference Sinibaldi and Ramirez12].
Regardless of the high seroprevalence found in women of childbearing age in Brazil, few data are available on the actual incidence of congenital toxoplasmosis. Existing studies show an incidence of 0·33–1·96/1000 live births, based on the detection of anti-toxoplasma IgM antibodies from newborn blood samples [Reference Carvalheiro13].
The aim of this study is to estimate the annual incidence of congenital toxoplasmosis occurring in São Paulo Metropolitan Region (SPMR), utilizing mathematical modelling, from 1984 to 2006, using seroprevalence data from a representative population.
MATERIAL AND METHODS
The community sampled
The community chosen for the seroprevalence study was the population of Caieiras, a city in SPMR. Caieiras has a population profile representative of the SPMR population, i.e. >90% live in the urban area, social structure is very heterogeneous and the economy is based on industries, trade and services.
Individuals stratified by age were randomly sampled within dwellings from administrative regions in a two-level cluster design. Out of the 62 administrative regions in the city, with 150 dwellings each, 32 were randomly selected. Further details on this population sampling have been published elsewhere [Reference Azevedo Neto14].
For the purpose of this study, the community sampled was divided in: infants aged 0–15 months (newborns in the first hour of life; 0·5 month; 1, 2, 3, …, 15 months); and individuals aged 1–39 years (1, 2, 3, 4, 5–9, 10–14, 15–19, 20–24, 25–29, 30–39 years). It was assumed 10 years of age onwards as possible for childbearing [Reference Leland15–Reference Baraldi17].
Blood was collected by a vacuum-containing system or by ‘butterfly needle’ venepuncture for children aged <2 years. Sera obtained after centrifugation of clotted samples were frozen and stored at −20°C for up to 5 years.
Serological data
To determine the presence of antibodies against T. gondii in each person, latex indirect agglutination (Toxoreagent; Eiken Co. Ltd, Japan) was used. This is a method for specific antibodies to this parasite in which latex particles are coated with toxoplasma antigen to form agglutinating patterns in the presence of specific antibody. The result was considered positive for antibody titres >1:32, according to the manufacturer's instructions. This serological method has a sensitivity and specificity of 89·6% and 91·4%, respectively [Reference Evans and Ho-Yen18].
SPMR statistics
Located in the Southeast Region of São Paulo state, Brazil, SPMR comprises approximately 10% of the Brazilian population, with 39 municipalities in an area of 8051 km2. SPMR is the largest urban conglomerate in South America, ranking among the largest megalopolises in the world. More details can be found in Fundação Seade database [19]. The SPMR population ranged from about 13 124 000 inhabitants in 1984 to almost 19 356 000 in 2006 [20].
From the Brazilian Statistics Institute (IBGE) database (1984–2006), the total number of deliveries D(y) (newborns and stillborns) of each year y was taken as the number of pregnant women per year. Deliveries are reported in relation to the parturient age group, i.e. <15, 15–19, 20–24, 25–29, 30–34 and 35–39 years. Details can be found in the IBGE database [21–23].
Data management
Serological data were used to estimate the proportion of seropositive infants (aged 0–15 months) and the proportion of seropositive individuals (aged 1–39 years). The respective 95% confidence intervals (CI) were calculated.
An exponential function was chosen to represent the seroprevalence profile for infants, fitted to the following equation [Reference Almeida24]
where M(a) is the proportion of seropositive children at age a, M 1 is the amplitude of the curve, k is the rate of change of the proportion, and M 0 is a fitting parameter.
To represent the seroprevalence profile of individuals aged 1–39 years, S +(a), serological data were fitted to the following the equation [Reference Farrington25]
where k 1 and k 2 are the parameters to be fitted [Reference Almeida24].
The age-dependent force of infection λ(a) is defined as the per capita rate in which susceptible individuals acquire infection per unit of time. In this study λ(a) was obtained from S +(a), from a catalytic model [Reference Muench26], according to
Assuming that the force of infection reaches a peak at an age which corresponds to a maximum number of infections, with a subsequent decline in higher ages, we can take the model [Reference Amaku27] modified from Farrington [Reference Farrington25]
The average age at infection can be estimated by [Reference Almeida24]
in which L was taken as 39 years, the maximum age observed from the serological data.
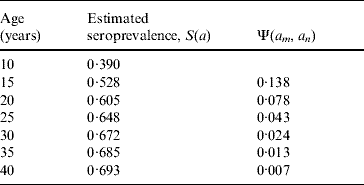
The increase in the proportion of seropositivity between ages a m and a n was used to calculate Ψ(a m, a n) by [Reference Valentim28, Reference Amaku and Azevedo29]
IBGE computes the number of births from women in age intervals a m to a n (10–14, 15–19, 20–24, 25–29, 30–34, 35–39 years).
The number of pregnant women in a given age group per year y,G(a m, a n; y), was then multiplied by Ψ(a m, a n), to estimate the number of new cases of toxoplasmosis in pregnant women, T(a m, a n; y), so
The average probability of vertical transmission of toxoplasmosis is 0·29 (95% CI 0·25–0·33), for any gestational period [Reference Dunn30], assumed as the probability of a congenital infection f. Then we may estimate the number of new congenital toxoplasmosis infections as
Finally we calculate the annual number of congenital toxoplasmosis cases per 1000 births E(y) by
This protocol was approved by the Ethics Committee (CAPPesq) of Hospital das Clínicas, Faculdade de Medicina da Universidade de São Paulo (process number 1090/07).
RESULTS
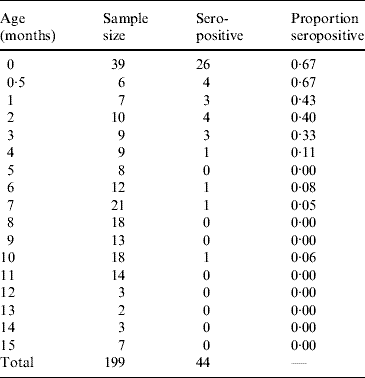
Table 1 presents the results obtained from the analysis of 199 infants and shows the proportion of seropositive infants M(a). Figure 1 shows the continuous function that represents the decrease in the proportion of seropositive infants M(a), as an effect of the decay of maternally derived antibodies. The fitting parameters for the infants' seroprevalence curve are M 0=−0·025 (s.e.=0·027), M 1=0·746 (s.e.=0·044) and k=0·299 month−1 (s.e.=0·046 month−1) (χ2=0·042, d.f.=14, P=1·0).
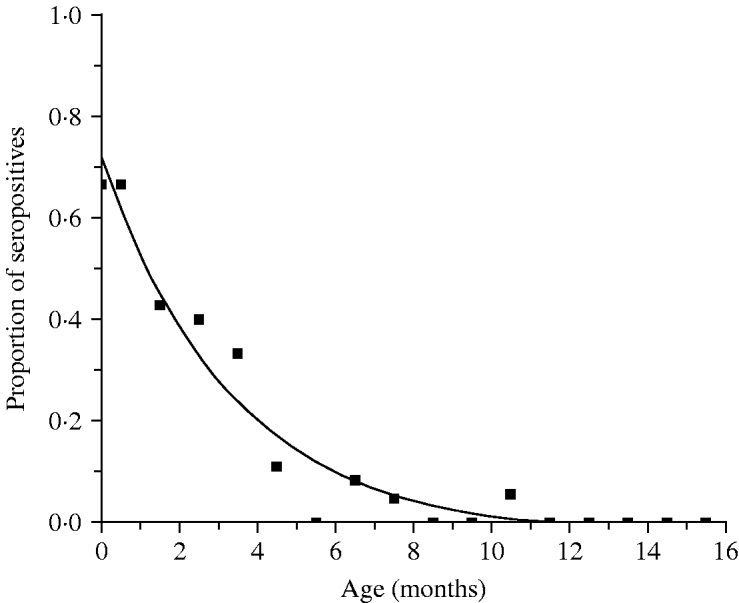
Fig. 1. Proportion seropositive M(a), and decay of maternal antibodies in children from 0 to 15 months, Caieiras, 1990. Fitting parameters: M 0=−0·025 (s.e.=0·027), M 1=0·746 (s.e.=0·044) and k=0·299 month−1 (s.e.=0·046 month−1).
Table 1. Proportion seropositive for toxoplasmosis from 199 infants aged 0–15 months, Caieiras, São Paulo state, Brazil, 1990
The overall positivity of toxoplasmosis in 250 sera samples from individuals aged 1–39 years was 26·8% (67/250). Table 2 shows the seroprevalence distributed by age group. The fitting parameters of the seroprevalence curve obtained were k 1=0·0237 yr−2 (s.e.=0·0031 yr−2) and k 2=0·140 yr−1 (s.e.=0·024 yr−1) (χ2=5·6, d.f.=8, P=0·7).
Table 2. Age-specific seroprevalence of toxoplasmosis in 250 individuals aged 1–39 years, Caieiras, São Paulo state, Brazil, 1990.
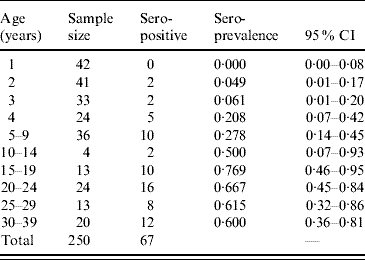
CI, Confidence interval.
Figure 2 shows the seroprevalence curve and the force of infection obtained from equation (3) of the community under study. The average age of acquisition of toxoplasmosis in this community, calculated using equation (4) was 10·74 yr (s.e.=0·71 yr).
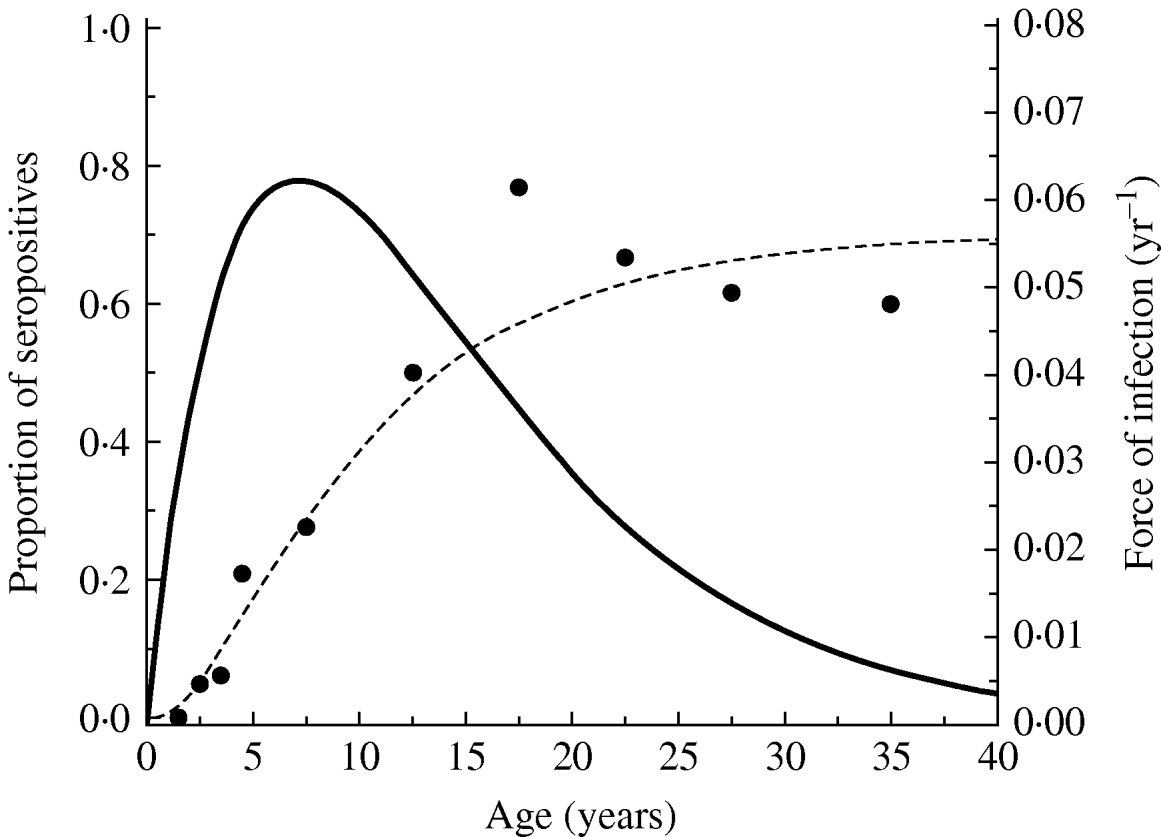
Fig. 2. Toxoplasmosis seroprevalence curve for Caieiras city, 1990. Fitting parameters: k 1=0·0237 yr−2 (s.e.=0·0031 yr−2) and k 2=0·140 yr−1 (s.e.=0·024 yr−1), and the force of infection obtained from equation (3) for the community under study.
Table 3 shows the increase in seroprevalence due to new infections Ψ(a m, a n) between ages a m and a n obtained from the seroprevalence curve. Figure 3 presents the number of deliveries for each maternal age group per year y, D(y). It can be observed there was an increase in the number of births in teenagers since 1984. Figure 4 shows the estimated number of congenital toxoplasmosis cases per year C(a m, a n; y) for each parturient age group, over the 23 years studied.
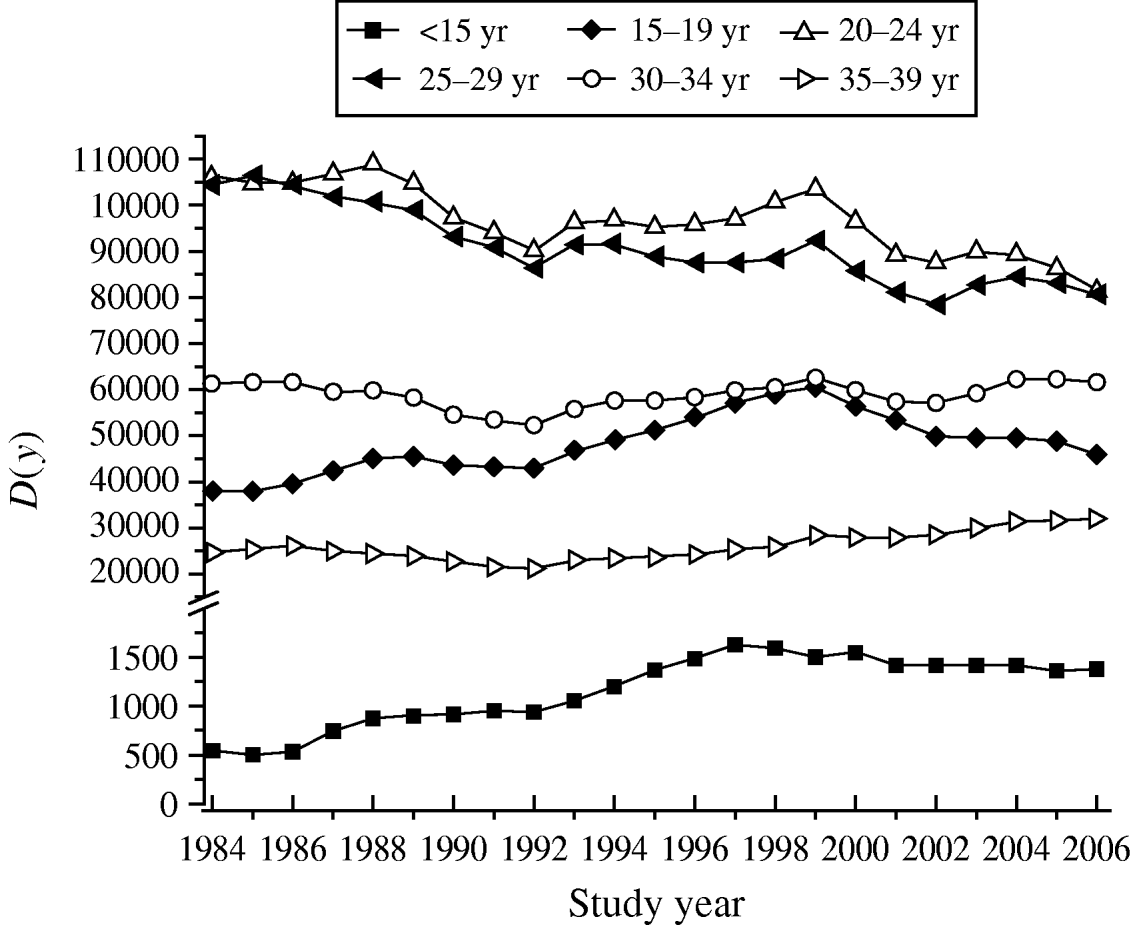
Fig. 3. Number of deliveries for each parturient age group per year y, D(y), from 1984 to 2006, São Paulo Metropolitan Region, Brazil.
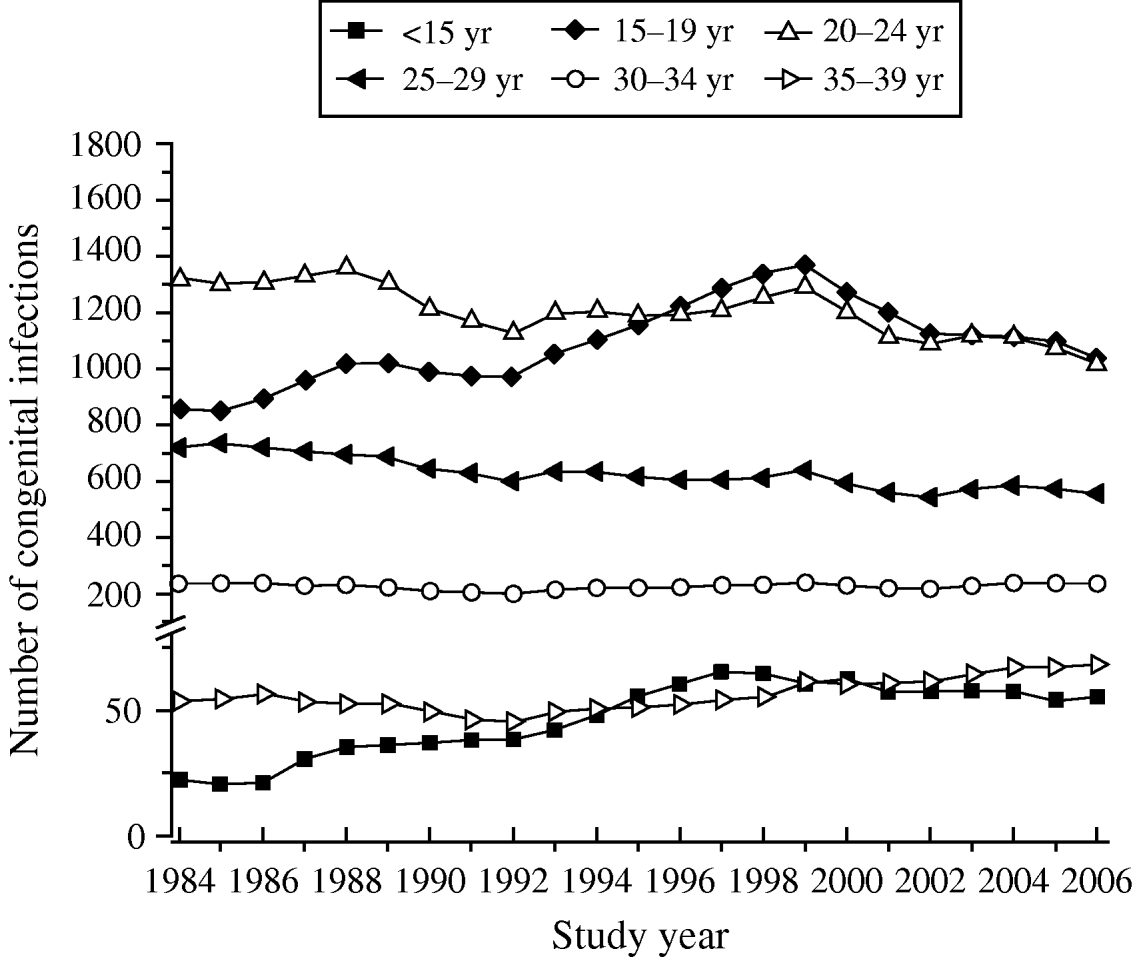
Fig. 4. Annual number of congenital infections, C(a m, a n; y), in each parturient age group, from 1984 to 2006, São Paulo Metropolitan Region, Brazil.
Table 3. Increase in the seroprevalence due to new infections between ages am and an, Ψ(am, an), obtained from the seroprevalence curve.
The estimated annual incidence of congenital toxoplasmosis E(y) in SPMR varied from 9·5 to 10·6 cases/1000 births in the study period.
DISCUSSION
The antibody prevalence estimate for T. gondii in women of childbearing age obtained in this study (64·9%) is in the range of previous studies with pregnant women and parturients in São Paulo state, in which prevalence varied from 51·5% to 67·4% [Reference Vaz31–Reference Galisteu34]. Sensitivity and specificity of assays used to estimate toxoplasmosis seroprevalence probably explains part of the variation found in studies.
The proportion of newborns with antibodies against T. gondii was 67%. This is in agreement with the serological findings for women of fertile age. The decay in the proportion of seropositive infants began in the first month of life, and, after 10 months of age it can be considered that maternal antibodies were cleared.
Mathematical modelling has been used previously to estimate incidence of toxoplasmosis in pregnant women. In France, from a seroprevalence of 60% for toxoplasmosis, published in previous studies, considering women aged between 15 and 44 years, Papoz et al. [Reference Papoz35] found 6·4 cases of congenital toxoplasmosis per 1000 newborns. However, the authors felt that the risk of acquiring infection is equal for all ages, from birth to death, and the seroconversion was not stratified by age, which may explain the difference in the number of cases compared to our study. On the other hand, Nokes et al. in Stockholm, Sweden, also used seroprevalence data to apply a catalytic infection model, in pregnant women aged 15–45 years old stratified by age, but in a longitudinal study recorded over 3 years, which may have provided a more accurate result that the one obtained in our study [Reference Nokes36].
Ades reviewed several studies in the UK on the estimation of primary infection during pregnancy by T. gondii and cytomegalovirus. He applied modelling on data obtained from these studies and proposed a method for calculating the number of women at risk, whereas the IgM persists during the same period of time for all. For toxoplasmosis, estimates of annual incidence cover sufficient variety, with significant heterogeneity within two extremes; however, the difference in this model and that used in our study makes it difficult to compare the results between them [Reference Ades37].
Finally, in Colombia, Gomez-Marin et al. [Reference Gomez-Marin, Montoya-de-Lonono and Castano-Osorio38], using two mathematical models previously developed [Reference Papoz35, Reference Ades37], estimated 7·1–10·6 new toxoplasmosis cases per 1000 pregnant women, rates lower than those found in our study.
Our model estimated congenital toxoplasmosis incidence between 9·5 and 10·6 cases/1000 births, a higher number when compared to some previously published data. This may reflect an overestimation of the model, although a Brazilian study demonstrated an estimate of eight cases of toxoplasmosis per 1000 live-birth infants from a public hospital [Reference Silva Segundo39].
The seroprevalence of toxoplasmosis in a representative community allowed us to estimate the congenital toxoplasmosis incidence in SPMR using mathematical modelling. A relatively simple model based on seroprevalence can provide coherent estimates for a congenital infection like toxoplasmosis in a given community, which is useful for health authorities in planning their actions towards prevention and control.
DECLARATION OF INTEREST
None.









