INTRODUCTION
Toxoplasma gondii infections in women of childbearing age are medically important because of the risk of transmission of the parasite to the foetus. Transplacental infection usually happens when a woman contracts T. gondii during pregnancy. The rate of congenital transmission and the degree of severity of toxoplasmosis in the foetus varies, depending largely on the gestational age at the time of infection; the risk of transmission is lowest in the first trimester and highest during the last trimester. Overall, the congenital transmission rate is about 40%. The highest risk to the foetus is when infection is acquired between weeks 10 and 24 of gestation. Most of the information on congenital toxoplasmosis is derived from prenatal screening of women in France for T. gondii infection. Seroprevalence in women has decreased markedly in France perhaps as a result of women becoming better educated regarding the danger of congenital toxoplasmosis [Reference Berger1]. Dubey [Reference Dubey2] and Pappas et al. [Reference Pappas, Roussos and Falagas3] summarized worldwide serological surveys during the last two decades for T. gondii antibodies in women of childbearing age, but none were listed for Portugal.
In Portugal, pregnant women are commonly tested for anti-T. gondii immunoglobulin M (IgM) and IgG in the first trimester of gestation, and again in the second and third trimesters if they are seronegative. Nevertheless, the predisposing factors of T. gondii infection are not completely elucidated and relatively little is known about its epidemiology. The aim of the present study was to assess the seroprevalence and evaluate associated risk factors of T. gondii infection in women from the North of Portugal in their childbearing years.
METHODS
Population sample
Between January 2009 and December 2010, 343 women attending gynaecology and obstetrics services of the public Hospital Centre of Trás-os-Montes e Alto Douro (CHTMAD), in Vila Real, and 58 women attending private clinics participated in this study. An individual questionnaire was completed for each woman. Confidentiality was maintained throughout the study period and the identity of participating subjects was available only to the medical staff involved.
Information sought included age, home town, residency in rural or urban area, type of housing (apartment or house), knowledge of toxoplasmosis, consumption of unwashed raw vegetables or fruit, meat consumption (beef, game, goat, lamb, pork, poultry and/or rabbit), consumption of raw or undercooked meat, consumption of processed pork products (cooked versus cured or smoked), performance of soil-related activities (agriculture or gardening) and possible use of gloves, and contact with cats (Table 1).
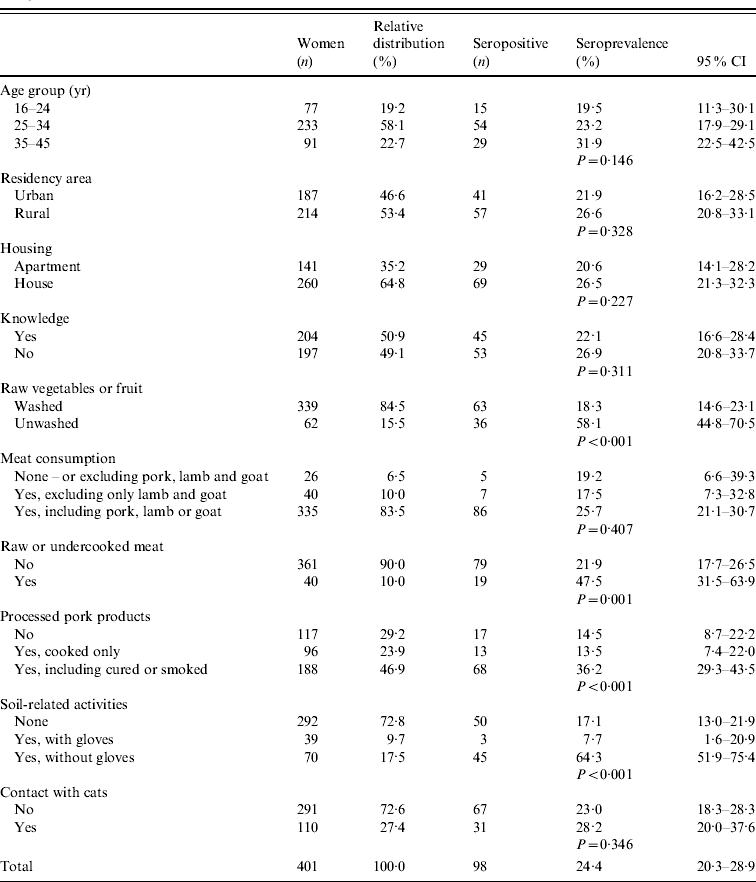
Table 1. Seroprevalence of Toxoplasma gondii infection in women of childbearing age according to independent categorical variables
CI, Confidence interval.
Serological testing
The results of specific IgM and IgG antibodies to T. gondii were obtained from medical records. Serology had been performed using a commercial chemiluminescent microparticle immunoassay (CMIA; qualitative Architec Toxo IgM® and quantitative Architec Toxo IgG®, Abbott Laboratories, Germany).
Data analysis
Seropositivity to T. gondii for the above-mentioned categorical variables were statistically compared using the χ2 test. Confidence limits for the proportions were established by exact binomial test with 95% confidence intervals (CIs). Variables showing a significant difference between groups (P<0·05) were selected for multiple logistic regression analysis to identify independent risk factors for seroprevalence, calculating odds ratios (ORs) and their 95% CIs. Statistical analyses were performed with SPSS 11.5 software for Windows (SPSS Inc. USA). For a population of 1 900 000 women in the North of Portugal, a default seroprevalence of 50% and a confidence level of 95%, an absolute error of 4·9% would be admissible by analysing a sample of 401 units [Reference Thrusfield4].
Ethical aspects
This study was approved by the ethics committee of CHTMAD as well as by the medical boards of the private clinics. The purpose and procedures of this investigation were explained to all participants, and informed consent was obtained from all the women studied.
RESULTS
Antibodies (IgG and IgM) to T. gondii were found in 98 (24·4%) of 401 women (Table 1). Of the seropositive women, 92 (93·9%, age range 19–45 years) had only IgG antibodies; two (2%) women aged 28 and 32 years had only IgM; and four (4·1%) women (age range 24–35 years) had both IgG and IgM. Seroprevalence results for the three established age groups as well as for other independent variables are given in Table 1.
In additional pairwise comparisons, no statistically significant differences were found between the group not consuming processed pork products and the group eating only cooked pork products (cooked ham or sausages) (P=0·993), and between the group not engaging in soil-related activities and that wearing gloves during such tasks (P=0·202). On the other hand, significant differences (P<0·001) were detected between the group not consuming processed pork products and that eating such products cured or smoked (non-cooked ham or sausages), between the group eating smoked or cured processed pork products and that eating only cooked pork products; between the group not engaging in soil-related activities and that not wearing gloves, and between the group not wearing gloves and that wearing gloves during such tasks.
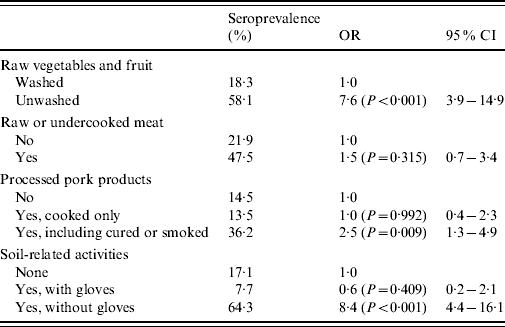
Risk factors for T. gondii infection in decreasing order were: soil-related activities without gloves (OR 8·4), consumption of unwashed raw vegetables or fruit (OR 7·6) and eating smoked or cured processed pork products (OR 2·5) (Table 2). Consumption of raw or undercooked meat was not identified as a risk factor.
Table 2. Identification of risk factors for Toxoplasma gondii infection in women of childbearing age (n=401) by multiple logistic regression
OR, Odds ratio; CI, confidence interval.
An association was found between soil-related activities without gloves and eating smoked or cured processed pork products (P<0·001), but not between soil-related activities without gloves and consumption of unwashed raw vegetables or fruit (P=0·330) or between consumption of unwashed raw vegetables or fruit and that of smoked or cured pork processed products (P=0·342).
DISCUSSION
The 24·4% seroprevalence in women found in the present study indicates that 75% of women of childbearing age are at risk of becoming infected with T. gondii. Most (93·9%) of the seropositive women in our study had only IgG antibodies, indicating past exposure to the parasite. Only 2·0% had IgM only, which generally denotes a currently active or recent infection [Reference Jones5]. During pregnancy the likelihood of transmission to the foetus is much lower in chronically infected women [Reference Montoya and Liesenfeld6].
The lack of statistically significant differences in the seroprevalence levels of three age groups: 16–24 years (19·5%), 25–34 years (23·2%) and 35–45 years (31·9%) (Table 1) suggests that many women become infected at a younger age. The finding of IgM-positive women at up to age 35 years suggests that primary infections also occur at more advanced ages.
A study of the general Portuguese population, between 1979 and 1980, demonstrated a seroprevalence of 47% in 1875 individuals [Reference Ângelo7]. More recently, a prevalence of 28% was found nationwide in 7362 parturient women assessed with different serological tests [Reference Machado8]. A decline in the seroprevalence of T. gondii infection has occurred in women from the North of Portugal, from 31·4% [Reference Machado8] to 24·4% in the present study, with the difference being statistically significant (P=0·028). This situation suggests a declining trend in T. gondii prevalence in the North of Portugal.
In the present study, almost 50% of the women studied did not have any knowledge regarding toxoplasmosis. In view of these circumstances, information regarding toxoplasmosis and advice on its prevention should continue to be promoted among women, expectant mothers, and the general population.
Women who ate raw or undercooked meat had a significantly different seroprevalence compared to women without such habits, but this variable was not confirmed as a risk factor by multiple logistic regression (Table 2). According to Kijlstra & Jongert [Reference Kijlstra and Jongert9], T. gondii infection is associated with the consumption of undercooked meat or meat products, and up to 50% of infections are transmitted by consumption of undercooked meat, making toxoplasmosis one of the clinically most important foodborne diseases in pregnant women [Reference Ogunmodede10].
In Europe and the USA, pork has generally been considered a major source of T. gondii infection to humans [Reference Tenter, Heckeroth and Weiss11, Reference Dubey12]. In the present study, a considerable higher seroprevalence of infection was observed in women that consumed cured or smoked (non-cooked) pork products compared to the group that did not eat pork and the group that only consumed cooked pork products. A report on the isolation of viable T. gondii from samples of ready-to-eat cured meat showed that curing methods may not kill all tissue cysts [Reference Lunden and Uggla13, Reference Warnekulasuriya, Johnson and Holliman14]. Methods of curing or smoking meat have not been standardized and there is a great deal of variation [Reference Dubey2].
In the present study, women who engaged in soil-related activities, e.g. gardening or agriculture, without wearing gloves had a considerably higher seroprevalence compared to women that did wear gloves or did not have any soil contact.
When gardening or performing other agricultural activities, failure to wear gloves and wash the hands properly before eating or touching the face may cause the hands to become contaminated with sporulated oocysts through touching contaminated soil or gardening implements.
In some environments, oocysts can remain viable for years [Reference Dubey15]. Therefore, all soil, sand and untreated water should be considered as a potential source of infection for humans; this knowledge might explain the higher frequency of infection in women engaging in soil-related activities without using gloves, compared to women not working with soil [Reference Kravetz and Federman16, Reference Jones17]. Every health educational programme for the prevention of congenital toxoplasmosis should focus on educating women of all ages to avoid direct contact with soil, by wearing gloves when farming or gardening and to adhere to strict hygienic practices afterwards.
Contact with soil and water, rather than direct contact with cats, seems to be a risk factor for infection. A multicentre case-control study conducted in Europe also failed to identify cats as a risk factor for seroconversion in women during pregnancy [Reference Cook18]. As T. gondii oocysts take 1–5 days to sporulate and become infectious [Reference Dubey, Miller and Frenkel19, Reference Dubey20], the risk of infection is minimal if cat litter is changed daily. Nevertheless, some published studies report contact with cats and their presence at home as a risk for seroconversion for all women, with a greater risk for adolescents and pregnant women [Reference Baril21, Reference Barbosa, Holand and Andrade-Neto22].
In the present study, the risk factors for T. gondii infection identified (in decreasing order of their OR) were: contact with soil without gloves, consumption of unwashed raw vegetables or fruit, and consumption of smoked or cured pork processed products. The risk factors identified here are slightly at variance with the European multicentre case-control study by Cook et al. [Reference Cook18] who reported eating undercooked, raw or cured meat contributed 30–63% of infections, and contact with soil to up to 17%. Meat consumption by the Portuguese population is frequent, whether well done or undercooked. However, it seems that exposure to the environment and food contaminated by oocysts, rather than tissue cysts in meat, are the most important risk factors associated with T. gondii infection in women from the North of the country.
Little is known concerning the prevalence of viable T. gondii in food animals in Portugal. In a limited survey, de Sousa et al. [Reference de Sousa23] isolated viable T. gondii from the tissue of 15 pigs (40·5%) from a slaughterhouse in northeastern Portugal. Viable T. gondii was also isolated from tissues of 16 free-ranging chickens (26·2%) from 18 farms in north- and central-western Portugal [Reference Dubey24]. Infection in free-ranging chickens is indicative of soil contamination because these chickens feed from ground. We have found 35·8% seroprevalence of T. gondii infection in 204 domestic cats [Reference Lopes, Cardoso and Rodrigues25] and 38·0% in 673 domestic dogs from northeastern Portugal [Reference Lopes26]; and 50% in 52 wild birds and 90% in 20 wild mammals from northern and central areas of the country [Reference Lopes27]. Taken together, these findings suggest a considerable presence of sporulated oocysts in the local environment.
In conclusion, most women of childbearing age from the North of Portugal are susceptible to primary infection with T. gondii and, therefore, the risk of congenital toxoplasmosis remains high. As such, continuing education about dietary and environmental sources of infection remains essential in the forthcoming years and effective preventive measures must be put into practice. Results provided by the present work are useful information to the medical and public health authorities when addressing policies for monitoring and controlling infection and disease in Portugal.
ACKNOWLEDGEMENTS
The authors thank the women who responded to the questionnaire for their cooperation. Special thanks are also due to Nurse Manuela P. Silva and Ms. Júlia Torres for their assistance. A. P. Lopes was supported by scholarship SFRH/BD/44438/2008 from Fundação para a Ciência e a Tecnologia, Portugal, and the European Social Fund.
DECLARATION OF INTEREST
None.




