INTRODUCTION
Pertussis, also known as whooping cough, is caused by the Gram-negative bacterium Bordetella pertussis and is a very contagious respiratory infection. Pertussis is characterized by severe coughing spasms often associated with vomiting and is particularly dangerous for infants [Reference Edwards1]. In the early 19th century the majority of the population was infected with pertussis before the age of 10 years, but this declined markedly when most European countries introduced universal child vaccination in the 1950s [Reference Pebody2]. Today, however, pertussis continues to be endemic both in countries with high and low vaccination coverage [Reference Guiso, Liese and Plotkin3]. During the last decades, the incidence of reported cases has increased worldwide, especially in adolescents and adults [Reference Pebody2, Reference Zepp4, Reference Quinn and McIntyre5]. An incidence of 4·1/100 000 persons per year was estimated from reported cases in 20 European countries during a 5-year period (2003–2007). Of all cases, 31% (13036 cases) were reported in people aged >20 years [6]. In Denmark, the incidence has ranged between 6 and 11/100 000 cases from 2006 to 2010 [Reference Dalby, Christiansen and Andersen7–Reference Dalby11]. Pertussis typically arises in an epidemic cycle every 3–5 years, and the latest epidemic in Denmark was in 2002 with an incidence of 36/100 000 persons. The two latest recorded deaths from pertussis occurred in 2005 and 2010; both were unvaccinated infants aged <2 months [Reference Dalby, Knudsen and Andersen9, Reference Pedersen, Fisker and Andersen12]. The overall incidence of reported cases in Denmark has not increased markedly during the last decades, but an increased incidence in older age groups has been reported.
Even though symptoms are most severe in infants, adults are important reservoirs for transmission of pertussis to unvaccinated or partially protected infants [Reference Wiley13]. Adults with pertussis can present with a mild course of the disease [Reference Yih14] and adult pertussis is therefore most likely under-diagnosed [Reference de Melker15].
Several factors are thought to play a role in the re-emergence of pertussis [Reference Wood and McIntyre16]. Protection from vaccination or infection is estimated to wane after 4–12 years and 4–20 years, respectively [Reference Wendelboe17]. This means that a large proportion of the adolescent and adult population is at risk of getting pertussis and transmitting the disease to infants, too young to be vaccinated. The observed increases in disease incidence around the world may also be attributed to improved diagnostic tests and improved awareness of the disease. Culture, polymerase chain reaction (PCR) and serology are the methods available for diagnosing B. pertussis infection. The methods have varying sensitivity and specificity mainly depending on the stage of the disease [Reference Wood and McIntyre16]. While culture and PCR are useful in the early course of the infection, serology has the ability to test for the presence of specific antibodies for a long period after infection. The use of serology could therefore reduce under- and misdiagnosis of patients, who tend to present late after a prolonged coughing illness. This is probably typical behaviour for adults. Furthermore, PCR and culture have lower sensitivity for adults than for children [Reference Crowcroft and Pebody18]. The recommended serological method for pertussis diagnosis involves the measurement of IgG antibodies against pertussis toxin (IgG anti-PT) [Reference Guiso19], and it has been estimated that increases in IgG anti-PT are measureable in over 90% of B. pertussis infections [Reference Wood and McIntyre16].
A serological diagnostic test for pertussis was introduced in Denmark in 2010 [Reference Dalby, Knudsen and Andersen9]. It is likely that the use of serology will result in a future increase in the numbers of correctly diagnosed pertussis cases. Recently, a study conducted in Denmark suggested that B. pertussis may be under-diagnosed in older children and adults with cough. Of 178 patients with reported duration of cough between 2 weeks and 3 months after blood donation, 6·4% had elevated levels of IgG anti-PT antibodies >75 IU/ml indicating recent pertussis infection [Reference Dalby20].
The diagnosis of pertussis in adults and adolescents is often delayed due to lack of classical symptoms and/or low physician awareness, whereby there is a risk of unknown transmission [Reference Wood and McIntyre16]. The awareness in Danish general practitioners regarding the burden of pertussis in adults is probably as low as found in a study conducted in European healthcare professionals in 2008 [Reference Hoffait21].
The purpose of the present study was to determine the seroprevalence of B. pertussis infections in a cross-sectional survey in a randomly selected adult population in the Copenhagen area, Denmark between 2006 and 2008. Furthermore, we aimed to estimate the seroincidence based on a back-calculation model and describe the risk factors associated with high levels of IgG anti-PT in the study population.
METHODS
Study population
Data were obtained from the population-based cross-sectional survey, Health2006, conducted in Denmark between June 2006 and June 2008 by the Research Centre for Prevention and Health (RCPH; www.fcfs.dk). A random sample of men and women aged 18–72 years from 11 municipalities in the south-western part of Copenhagen were selected through the Danish Civil Registration system. Pregnant women were excluded based on a precautionary principle because the examination included various allergy tests such as patch tests and skin prick tests.
Written consent was obtained from all participants, and the study was approved by the local ethics committee as described elsewhere [Reference Thuesen22].
Of 7931 individuals invited, 3471 agreed to participate (response rate 44·7%). The participants completed a questionnaire concerning their physical and mental well-being, socio-demographic conditions and different health behaviours. Next, they underwent an extensive health examination and serum samples were obtained from a total of 3440 participants (1895 women, 1545 men), defining the population eligible for analyses. The reasons for the 31 (<1%) missing blood samples were mainly difficulties with blood drawing.
The participation rate was higher in women and older age groups. The study area was overall representative for the capital region of Denmark except for a slightly lower social status [Reference Thuesen22].
Determination of pertussis antibodies
Levels of IgG anti-PT were measured in serum samples from the 3440 participants by indirect enzyme-linked immunosorbent assay (ELISA) as described previously [Reference Dalby23]. Results are expressed as international units (IU) per ml according to the WHO International Standard Pertussis Antiserum (National Institute for Biological Standards and Control, Potters Bar, UK, code 06/140). Pertussis toxin is specific for B. pertussis and there are no known antibody cross-reactions to antigens from other bacteria [Reference de Melker24].
A cut-off level of 75 IU/ml was chosen, as this threshold was recently found to be optimal in sensitivity and specificity as a diagnostic cut-off for single-sample serology in the Danish population [Reference Dalby, Harboe and Krogfelt25]. Seroprevalence will, however, also be reported according to cut-off values of 50 IU/ml and 125 IU/ml as suggested by the EU Pertstrain Group [Reference Guiso19] in order to compare the results with past and future studies. A cut-off of 125 IU/ml has been estimated to be indicative of recent or active infection, while a cut-off of 50 IU/ml may be especially useful in outbreak situations [Reference de Greeff26].
In Denmark, there are no current recommendations for adult pertussis boosters [27], and elevated levels of IgG anti-PT antibodies in sera from adults are therefore considered to be due to infection rather than vaccination.
Pertussis antibody decay profiles determined by longitudinal data
To estimate the time since last pertussis infection we used longitudinal data from a previous Danish study including antibody decay profiles from 71 persons with bacteriologically confirmed pertussis infection. A detailed description is available elsewhere [Reference Dalby28].
Statistical analysis
The age-specific prevalence was calculated for the diagnostic cut-off value 75 IU/ml, as well as the values 50 IU/ml and 125 IU/ml. Direct age-standardization of the prevalences was undertaken using the age structure of the population in Denmark in July 2007.
To determine whether there are risk factors associated with an increased risk of pertussis infection, we conducted univariable and multivariable logistic regression analysis. The analyses were performed for the following factors: gender, age (19–29, 30–39, 40–49, 50–59, 60–72 years), education [none, <1 year, craft education, <3 years (short), 3–4 years (medium), >4 years (long)], employment status (employed, formerly/never employed), type of residence (apartment, house, terrace house, other), living area (<70, 70–110, >110 m2) and number of persons in home (1, 2–3, 4–12 persons), smoking status (smoker, ex-smoker, never), alcohol consumption (0, 1–13, 14–21, >21 drinks/week) and asthma (‘Has a doctor ever told you that you have asthma?’). Reported symptoms of coughing (‘Do you usually cough during the day/night?’ and ‘Do you cough in this way most days, around 3 months a year?’) were also tested but not included in the analyses. Odds ratios (ORs) are presented with 95% confidence intervals (CIs).
A test for seasonal variation in pertussis infection was conducted using Serfling's method [Reference Serfling29]. The seroincidence was calculated using a back-calculation model to estimate the time between blood sampling and infection when fitted to the longitudinal data [Reference Simonsen30]. By using the decay rate, we then estimated the time since last infection for each individual from the cross-sectional data and the seroincidences were calculated.
All analyses were performed with the statistical program SAS versions 9.2 and 9.3 (SAS Institute Inc., USA). The P values reported are all two-tailed with statistical significance defined as P < 0·05.
RESULTS
Seroprevalence and seroincidence
A total of 3440 serum samples collected between June 2006 and May 2008 were tested for IgG anti-PT. The median age of the participants was 50 years and 55% were women.
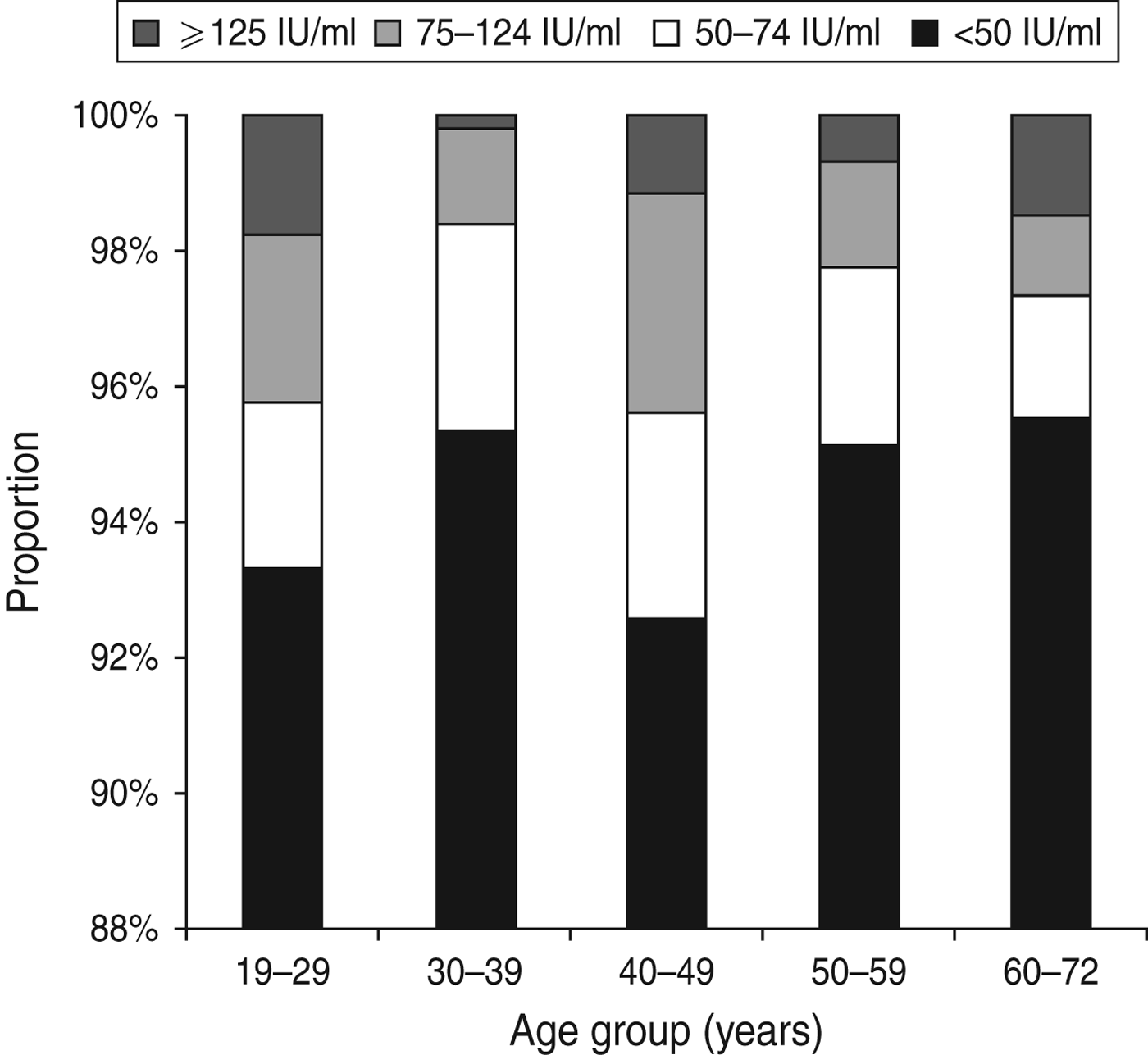
The IgG anti-PT level in the study population had a median of 8·7 IU/ml ranging from 1·6 to 300 IU/ml. Overall, 102 persons had an IgG anti-PT concentration ⩾75 IU/ml corresponding to an age-standardized seroprevalence of 3·0% (95% CI 1·9–4·7). For the cut-off values 50 IU/ml and 125 IU/ml, the age-standardized seroprevalence was 5·6% (95% CI 4·1–7·8) and 1·1% (95% CI 0·5–2·3), respectively. The age-specific seroprevalences according to the three cut-off values are presented in Figure 1.
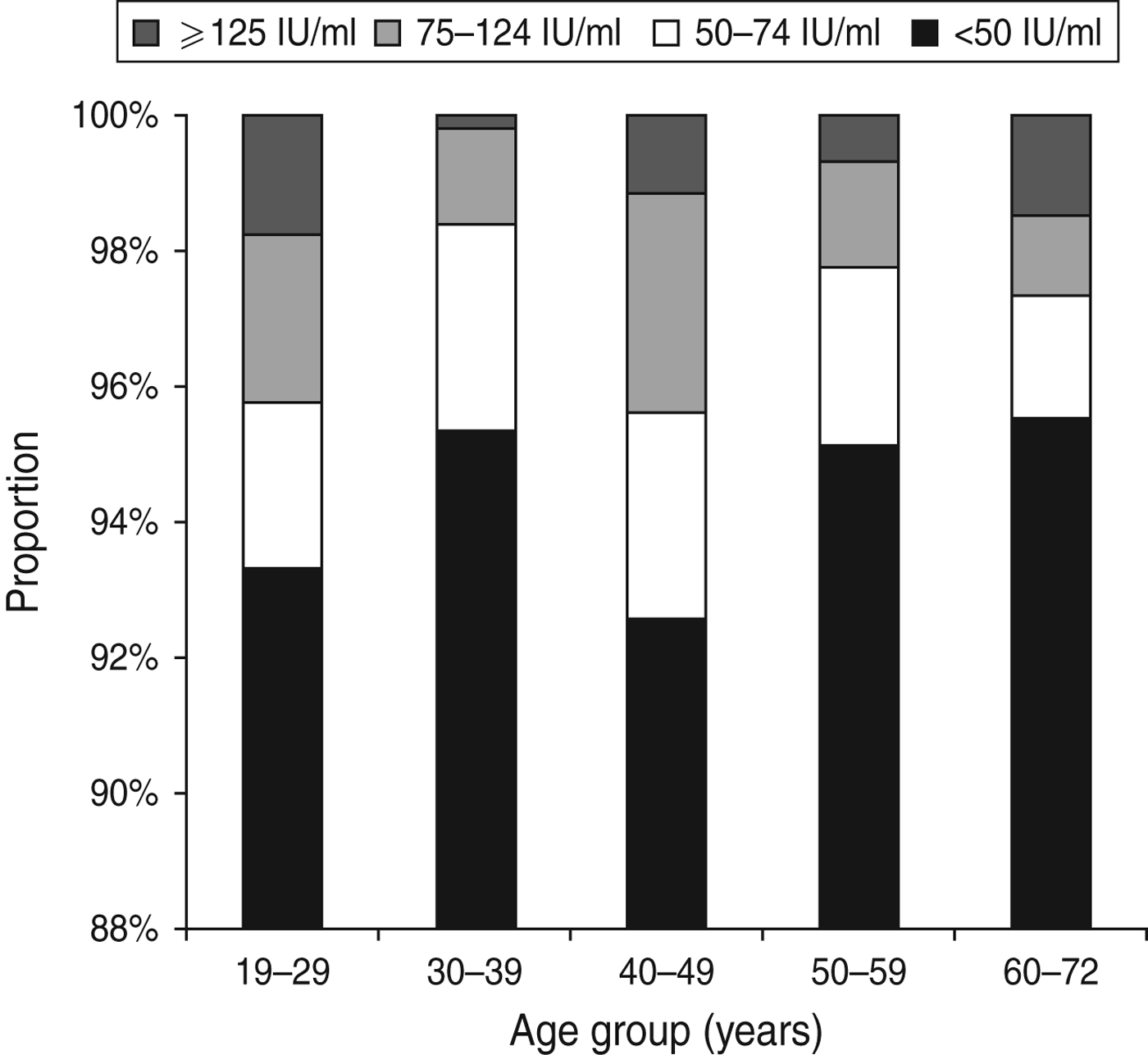
Fig. 1. Age-specific distribution of IgG anti-PT levels.
Using data from the 71 patients in the longitudinal study we estimated parameters, which determined the long-time decay of IgG anti-PT level after onset of pertussis. From these parameter estimates we predicted a halftime of about 170 days. When the parameters were used to back-calculate the measurements from the cross-sectional sample from Health2006 into estimates of time since last infection we estimated a seroincidence of 0·143/person-years (95% CI 0·131–0·155) or 143/1000 person-years for an IgG anti-PT level ⩾75 IU/ml. There was no marked difference across age groups. By comparison, a total of 233 notified laboratory-confirmed cases (by PCR and/or culture) were found in the adult population (19–72 years) during the same period (2006–2008), this corresponds to a pertussis incidence of 0·031/1000 person-years.
The seroincidence for Health2006 for the cut-off value 75 IU/ml compared to the incidence of the notified cases according to age group is shown in Figure 2.
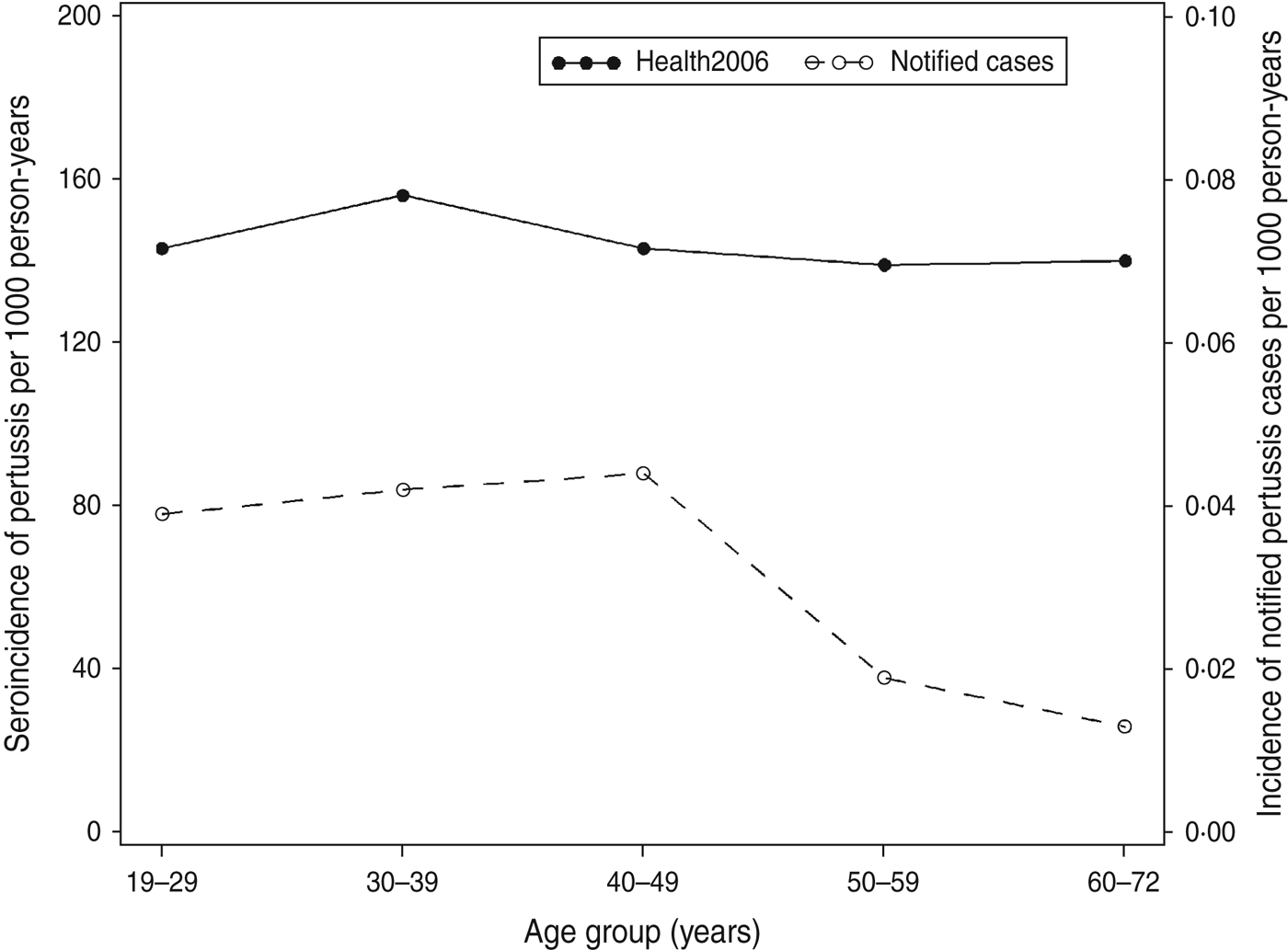
Fig. 2. Estimated incidence of pertussis in Health2006 per age group (filled circles, left scale) and the incidence of notified pertussis cases in Denmark in 2006–2008 per age group (open circles, right scale).
Risk factors
Results from the univariable and multivariable analyses of the different risk factors are shown for the diagnostic cut-off of 75 IU/ml in Table 1. Men showed higher levels of IgG anti-PT than women, this was, however, not statistically significant. There was a statistically significant difference in IgG anti-PT level between age groups with a decreased risk of infection in the 50–59 years group (OR 0·36, 95% CI 0·14–0·87) compared to the reference group (19–29 years) after adjusting for other factors. According to education there was an increased risk in those with a long education compared to people with no education although this was not significant (OR 1·64, 95% CI 0·78–3·46). There was no association between the prevalence of high titre IgG anti-PT concentrations and employment status, type of residence, living area, number of persons in home, smoking status, alcohol consumption, or asthma (Table 1). Neither did symptoms of cough show any significant association with pertussis.
Table 1. Risk factors for high titres (⩾75 IU/ml) in sera collected in Health2006 between 2006 and 2008
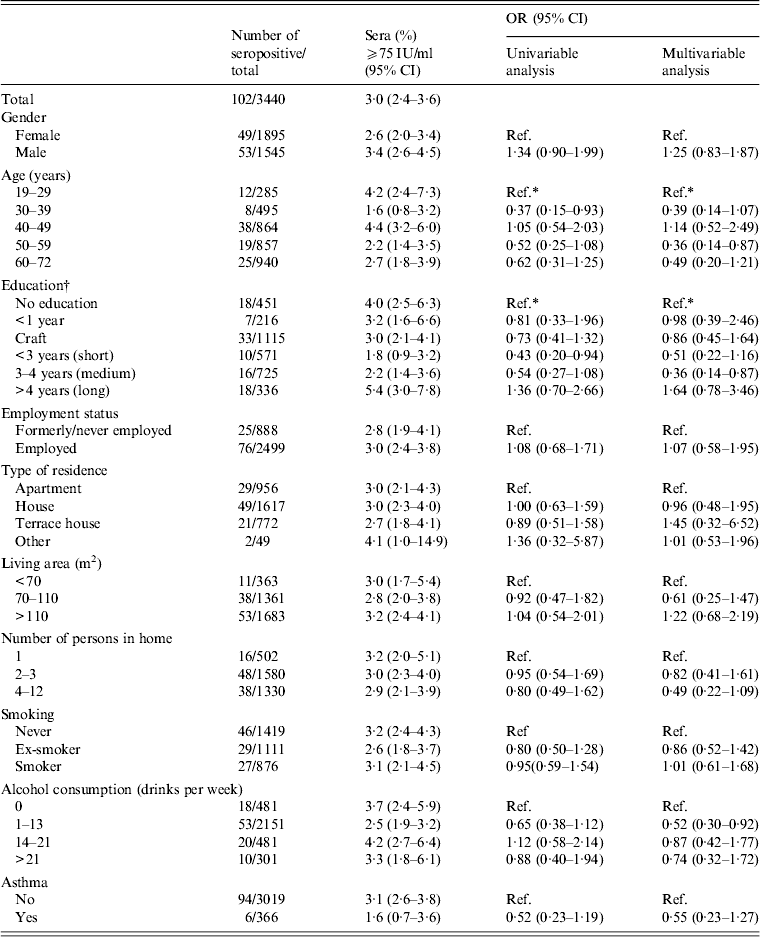
OR, Odds ratio; CI, confidence interval.
* Significant (P < 0·05).
† Education is based on type and length of education after primary school (<16 years). If education was marked as ‘other’ it was either classified after length of education or as missing.
During the sampling period there were no indications of an outbreak situation as the IgG anti-PT concentrations are distributed evenly across the period (Fig. 3). Furthermore, there was no significant seasonal variation observed in pertussis infection when tested by Serfling's method (P = 0·857).
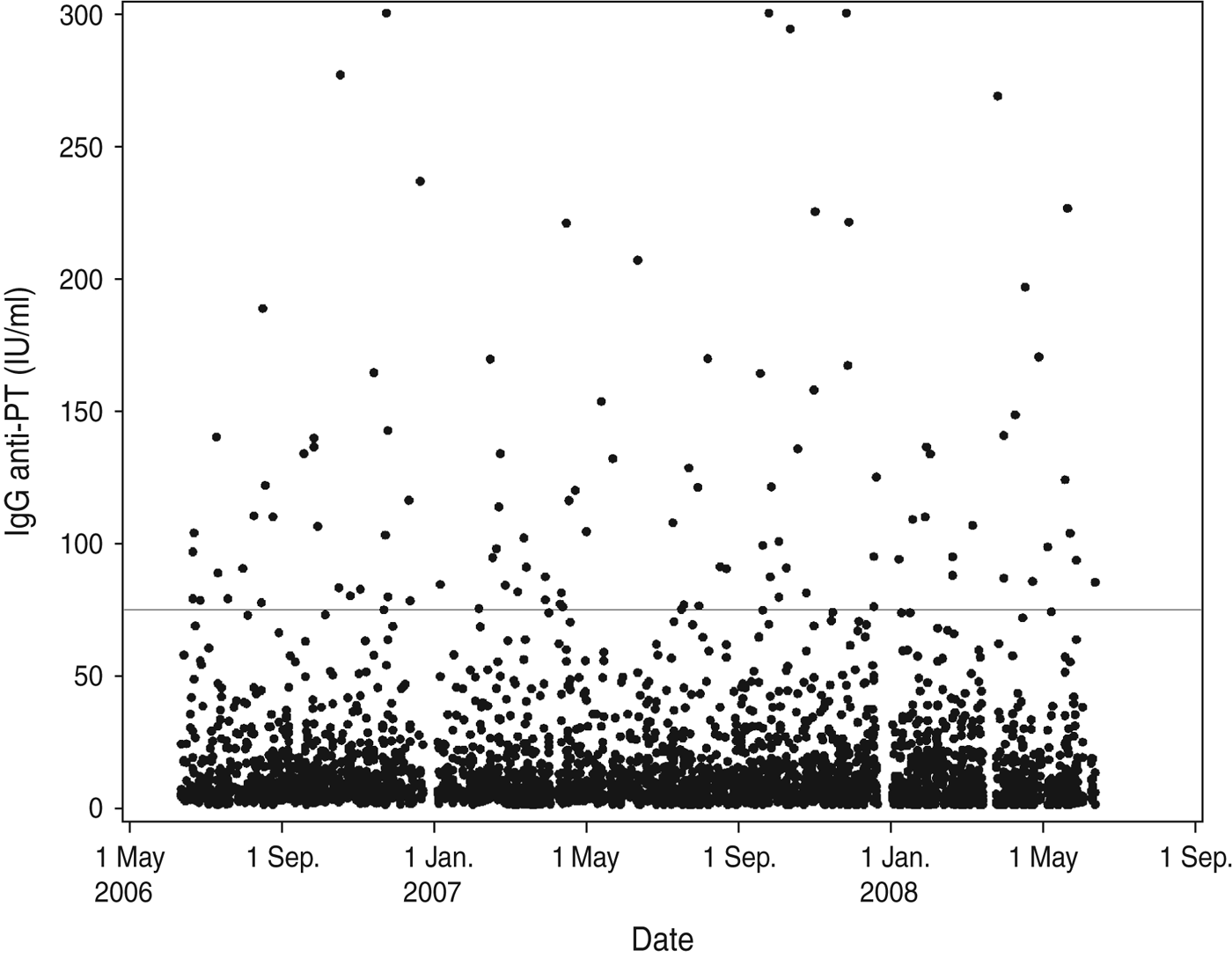
Fig. 3. Distribution of IgG anti-PT results according to date of blood-sample donation. The horizontal line indicates the diagnostic cut-off value 75 IU/ml.
DISCUSSION
With an overall seroprevalence of 3·0% in Health 2006 and an age-specific seroprevalence ranging from 1·62% to 4·4% (⩾75 IU/ml), this study indicates that B. pertussis is under-diagnosed in the Danish population. In the study, 1·1% of the population had indications of recent infection (⩾125 IU/ml), which is consistent with seroprevalences found in adult populations in other countries [Reference de Greeff31, Reference Rendi-Wagner32].
The estimated halftime of 170 days was consistent with the Dutch studies by de Melker and colleagues [Reference de Melker15, Reference de Melker24]. The incidence of pertussis in Health2006 was estimated to be 143/1000 person-years in adults. This is 4613 times higher than the incidence of the notified pertussis cases on 0·031/1000 person-years reported in the same period. This difference indicates that pertussis is highly underestimated in adults in Denmark. In other studies, underestimation of pertussis has similarly been suggested [Reference de Melker15, Reference Rendi-Wagner32, Reference Kretzschmar, Teunis and Pebody33]. A prospective study of Australian adults (>45 years) recently reported an incidence rate of 94 notified cases/100 000 [Reference Liu34]. In The Netherlands an estimated incidence of pertussis of 6·6% was found for a cut-off of 50 IU/ml, which was 685 times higher than the notified cases [Reference de Melker15]. The study was, however, conducted in 3- to 79-year-olds in 1994–1996, which might explain some of the discrepancy with our findings, as well as a different history of vaccination and awareness of pertussis. In a study of five European countries a seroincidence between 1% and 6% was reported using a similar back-calculation approach [Reference Kretzschmar, Teunis and Pebody33]. Inconsistency with other studies may in general be influenced by the use of different methods, for instance diagnostic cut-off, as this varies between countries.
The large discrepancy between the notified cases and serologically diagnosed cases in our study can be due to several factors. First, the notified cases depend on the level of medical care-seeking, which could vary according to age and gender. Of the clinically notified cases (based on culture and PCR) a higher incidence in women than men has been observed [Reference Dalby, Christiansen and Andersen7, Reference Dalby10, Reference Dalby11]. The sex difference in cases confirmed in 2008 increased with age and the incidence was three times higher in women than men in the 30–39 years age group [Reference Dalby, Christiansen and Andersen7], which is contrary to the serologically diagnosed cases in our study. This difference could be explained by the fact that men seek medical care less frequently than women [Reference Addis and Mahalik35] and much later in the course of the infection, when PCR and culture would test negative (serology was not available as a diagnostic test in Denmark during the study period). The reason for the higher IgG concentrations in men tested by serology is, however, unknown. It could be caused by an unknown biological mechanism making men more susceptible, or some social differences between the participating men and women.
Second, a great part of the serologically detected infections may represent asymptomatic cases. It has been estimated that asymptomatic cases in adolescents and adults are five times more common than clinical illnesses, thus some of these cases might be an important source of transmission [Reference de Greeff31]. This study emphasizes that the use of serology provides a better opportunity to detect such asymptomatic cases, and gives a better estimate of the actual number of infected persons. Knowledge about the role of asymptomatic cases is inconsistent. One study suggest that 16% of infections in infants are the result of transmission of asymptomatic disease [Reference Wendelboe36], while another study indicates that they contribute little, if anything, to pertussis transmission [Reference Wiley13].
Most of the risk factors investigated in our study are intended to represent socioeconomic status. The knowledge about the role of social status in the risk of pertussis is very limited. Two previous seroepidemiological studies have investigated the role of socioeconomic status on the risk of pertussis. Both studies found that people with low income were more likely to have high IgG anti-PT concentrations [Reference de Greeff31, Reference Rendi-Wagner32]. Less educated, poor, smokers, and individuals with asthma, cancer or diabetes, had increased risks of pneumococcal pneumonia in one study [Reference Flory37], these could also be factors in the transmission of pertussis. Contrary to our hypothesis, the study did show a significant difference between educational level and pertussis, but individuals with the highest educational level had a 64% increased risk of pertussis (⩾75 IU/ml) compared to people with no education, while the other education groups had a lower risk. We found no association between the other socioeconomic factors. The estimates have, however, overlapping confidence intervals, and caution should therefore be excercised before making conclusions. An alternative explanation could be that people from higher social status have more social contacts and therefore higher risk of contracting the infection [Reference Charland38]. Information on income was not available in Health2006, which could have been relevant in order to fully assess the impact of socioeconomic status, especially when considering findings from other studies [Reference de Greeff31, Reference Rendi-Wagner32].
Alcohol, smoking and asthma were investigated under the possible hypothesis that these factors could make people more susceptible to respiratory system infections. Smoking has substantial local and systemic effects on the respiratory tract, the immune system and soft tissues, which could alter both the susceptibility to infection and the course of an infectious disease, e.g. pneumonia, tuberculosis, and influenza [Reference Arcavi and Benowitz39, Reference Huttunen, Heikkinen and Syrjanen40]. Alcohol has similarly been associated with an increased risk of pneumonia, even as a dose–response relationship [Reference Samokhvalov, Irving and Rehm41]. Moreover, an increased risk of pertussis has been found in patients with asthma in previous studies [Reference Liu34, Reference Capili42] and asthma was estimated to comprise 17% of the risk of pertussis in one study [Reference Capili42]. Neither alcohol, smoking or asthma were associated with pertussis in our study (Table 1). More research is needed to investigate those associations conducted as follow-up studies in which information on the exposure is collected prior to index date of pertussis infection.
We acknowledge that there are some limitations to our study. Due to the relatively low participation rate (44·7%) in Health2006, the generalizability of the results is reduced. There is a risk of sampling bias because of a higher level of participation in women and older age groups [Reference Thuesen22]. Furthermore, it is possible that people with pronounced symptoms from pertussis omit participating in the study due to the inconvenience of the sudden cough attacks and vomiting.. The estimated seroincidence should be interpreted with caution because of great individual variation in IgG anti-PT concentrations. The model did, however, incorporate the individual variation in antibody response and the fact that antibody responses fall to a steady-state level after an infection course and do not approximate zero.
We have no information on vaccination status of the participants but the IgG anti-PT concentrations measured in this study indicates post-infection rather than post-vaccination antibodies since there are currently no recommendations for adolescents or adult booster vaccinations for pertussis in Denmark. Furthermore, none of the participants (all aged >18 years in 2006) were eligible for the pertussis preschool booster which was introduced in 2003 [Reference Anderson and Stellfeld43]. Hence, the participants were given their last pertussis vaccination, if any, when they were 1 year old.
The results from our study are of public health importance because pertussis continues to circulate silently in highly vaccinated populations, transmitting the disease to vulnerable subjects. In particular, the risk of transmission to non-immune infants is of great public health concern and is the primary reason for contemplating additional vaccination strategies, as seen in several countries [Reference Pebody2, Reference Zepp4]. The results also emphasize the importance of using serology both as a diagnostic method and as a tool to determine the epidemiology of pertussis in the population. The observed difference between notified cases and those serologically diagnosed in this study could, furthermore, indicate the need to raise awareness and increase information about the burden of pertussis, especially in adult populations, to general practitioners and the general population The knowledge of potential risk factors is valuable when considering immunization against pertussis for adult populations. A tetanus-diphteria-pertussis vaccine, previously used for the preschool booster in Denmark (TdaP vaccine, SSI) has recently been tested in a Danish clinical trial and approved for use in adults, making immunization of the adult population in Denmark a possible prevention strategy in the future [Reference Thierry-Carstensen44].
ACKNOWLEDGEMENTS
We are very grateful for the laboratory assistance provided by the Serology Laboratory, Department of Microbiological Diagnostics & Virology, Statens Serum Institut.
DECLARATION OF INTEREST
None.