One-third of the world's population is currently infected with Mycobacterium tuberculosis, and each year more than 1·5 million people die as a result of this disease [1]. Tuberculous meningitis (TBM) is the most severe extrapulmonary complication of tuberculosis and occurs mainly during early childhood. Haematogenous spread of bacilli from a primary pulmonary focus can lead to the development of a caseous granuloma in the meninges or brain adjacent to the meninges or ventricular ependyma. Rupture of this so-called ‘Rich focus’ into the subarachnoid space causes the clinical features of TBM. In the majority of cases, TBM develops within a few months after the primary infection [Reference Wallgren2].
Seasonal variation in the incidence of disease caused by M. tuberculosis (including TBM) has been described in the literature, but the exact cause of this phenomenon remains unknown [Reference Schaaf3, Reference Fares4]. Intensified transmission during winter periods, under conditions of overcrowded, poorly ventilated housing, might play a role [Reference Schaaf3]. Vitamin D deficiency, which is known to be associated with progression of M. tuberculosis disease [Reference Talat5], has also been suggested to play a role [Reference Fares4]. However, a possible association between vitamin D deficiency and TBM has never been evaluated. If vitamin D deficiency does play a role in the pathophysiology of TBM, an association between sunshine hours during the period before manifestation of the disease and the incidence rate of TBM would be expected.
Consecutive children aged between 6 months and 13 years, diagnosed with ‘definite’ or ‘probable’ TBM at a large tertiary teaching hospital in Cape Town, South Africa between April 2000 and April 2005, were included retrospectively in the present study. Clinical data on these children were derived from a TBM study database published by van Well et al. [Reference van Well6]. A ‘definite’ diagnosis of TBM was made when M. tuberculosis was isolated from the cerebrospinal fluid (CSF). A ‘probable’ diagnosis of TBM was made when there were clinical signs of meningitis in the presence of characteristic CSF findings (macroscopically clear, pleiocytosis, elevated protein, reduced glucose) and two or more of the following criteria were present:
• recent poor weight gain;
• household contact with sputum smear-positive tuberculosis;
• computed tomography scan compatible with TBM;
• chest radiography compatible with primary tuberculosis;
• positive tuberculin skin test;
• acid-fast bacilli cultured from other clinical specimens [Reference van Well6].
The dates of admission were used to estimate the monthly incidence rate of TBM. Monthly sunshine hours between April 2000 and April 2005 were obtained from the South African Weather Service and used for analysis. Sunshine hours were measured with a Campbell–Stokes sunshine recorder at Cape Town International Airport (coordinates: 33·97° S, 18·60° E; 42 m above sea level). Sunshine hours were defined as the total sum of hours during a certain period of time when the direct solar radiance exceeded 70 W/m2 in very dry air and 280 W/m2 in very humid air. Ultraviolet-B (UVB) radiation (280–315 nm) was measured with a Solar® Light UV Biometer, model 501 (Solar Light, USA) at the Global Atmosphere Watch (GAW) station, Cape Point (coordinates: 34·35° S, 18·49° E; 230 m above sea level) between February 2001 and April 2005. The UV biometer used is a meteorological grade instrument that measures biologically effective UVB radiation outdoors. Monthly UVB radiation was expressed in terms of the minimal erythema dose (MED); 1 MED is defined as 583 W/m2 falling continuously for 1 h.
To study the impact of monthly sunshine hours and UVB radiation on the incidence rate of TBM, the log-linear Poisson regression model was used. Incidence rate ratios (IRRs) with accompanying 95% confidence intervals (95% CI) were used as measures of relative risk. Time intervals of 1 month, counted from the month of admission backwards to 6 months before admission, were used for the analysis. Statistical significance was determined at the 1% level. Patient characteristics [sex, age, population group, HIV status, bacillus Calmette-Guérin (BCG) scar] were considered as potential confounding factors. The patients were divided into three population groups: white (European descent), black (African descent), and coloured (people of mixed descent, including Asiatic descent). Confounders that led to a change in the coefficient of more than 10% were added to the model. The Statistical Package for the Social Sciences version 18.0 for Macintosh (IBM®, USA) was used for the statistical analysis.
In total, 189 children aged between 6 months and 13 years were diagnosed with TBM between April 2000 and April 2005. The characteristics of the patients are summarized in Table 1. The highest number of sunshine hours was measured during the summer season (December–February), with a maximum of 361·90 h/month. The lowest number of sunshine hours was measured during the winter months (June–August), with a minimum of 165·50 h/month. The level of UVB radiation was comparable to the hours of sunshine during the summer season (maximum 335·86 MED/month), but during the winter months the level of UVB radiation showed a steeper decline than that of sunshine hours (minimum 20·29 MED/month).
Table 1. Characteristics of children admitted with a diagnosis of tuberculous meningitis (TBM) between April 2000 and April 2005
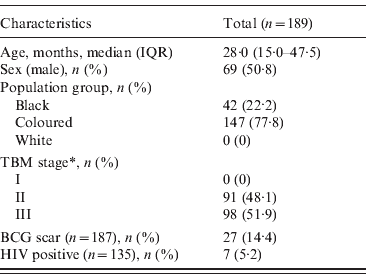
IQR, Interquartile range; BCG, bacillus Calmette-Guérin.
* TBM was staged using the modified criteria of the British Medical Research Council to determine the severity of TBM: stage I TBM [Glasgow coma score (GCS) 15 with no focal neurological signs]; stage II TBM (GCS 11–14 or GCS 15 with focal neurological deficit); stage III TBM (GCS <11).
A significant association was found between the monthly incidence rate of TBM and the number of hours of sunshine 3 months earlier (IRR per 100 sunshine hours 0·69, 95% CI 0·54–0·88, P = 0·002). This implies that a decrease of 100 sunshine hours/month was associated with a 45·0% (=1/0·69) increase in TBM incidence 3 months later (Fig. 1). Similar results were found for UVB radiation (IRR per 100 MED 0·75, 95% CI 0·61–0·91, P = 0·003).

Fig. 1 [colour online]. Incidence rate of tuberculous meningitis (TBM) (dashed curve; right y-axis) and sunshine hours (SH; dotted curve; left y-axis) are shown per quarter (=3 months). The solid curve (postponed sunshine hours) illustrates the inverse association with the incidence rate of TBM. Low amounts of sunshine during winter months lead to an increase in TBM incidence (black arrows). For reasons of clarity only part of the graph is shown.
This is the first study to demonstrate an association between the incidence rate of TBM and sunshine hours. Low amounts of sunshine during the winter months were associated with an increase in TBM incidence 3 months later. This finding suggests a possible role for vitamin D in the pathophysiology of TBM.
In the presence of UVB radiation, vitamin D3 is synthesized from a precursor in the skin (7-dehydrocholesterol). In general, solar UVB radiation is expressed in terms of the MED, which is defined as the dose of UVB irradiation which, after falling continuously, will start to damage human skin. The MED is a personal value and is dependent on age and skin type. During the winter months in Cape Town, the intensity of UVB radiation declines to about 1 MED a day, which can lead to insufficient vitamin D production, especially in persons with dark pigmented skin [Reference Armas7–Reference Martineau9].
The active form of vitamin D, 1·25-dihydroxyvitamin D3 (1·25D), has physiological functions that extend beyond its classical role in calcium homeostasis and bone metabolism. Over the last two decades, vitamin D deficiency has been found to be associated strongly with inflammatory diseases and those of long latency, such as multiple sclerosis, rheumatoid arthritis, diabetes, and M. tuberculosis disease [Reference Talat5, Reference Wagner, Taylor and Hollis10]. Once converted, 1·25D binds to the intracellular vitamin D receptor (VDR). This nuclear receptor is found in several cells that are involved in the human immune system, including stimulated macrophages, T cells, and B cells. Activation of the VDR influences patterns of cytokine secretion, suppresses T-cell activation, and can enhance the phagocytic activity of macrophages [Reference Liu and Modlin11].
Activation of monocytes by M. tuberculosis through the Toll-like receptor can lead to VDR-dependent production of pro- and anti-inflammatory cytokines and the expression of antimicrobial peptides, such as cathelicidin, that have direct antimicrobial activity [Reference Liu and Modlin11]. Recently, it was demonstrated that the production of pro-inflammatory cytokines, such as tumour necrosis factor-α, interleukin (IL)-6, IL-1β, and interferon-γ, shows seasonal variation and is dependent on vitamin D [Reference Khoo12]. Martineau et al. showed that there is a reciprocal seasonal relationship between serum vitamin D concentration and tuberculosis notifications in Cape Town, South Africa and concluded that seasonal variations in vitamin D status and tuberculosis incidence are directly causally related [Reference Martineau9].
Until now no studies have been published on vitamin D and its role in the pathophysiology of TBM. However, it is known that microglial cells, which play a key role in TBM, express VDR on the surface of the nucleus [Reference Eyles13]. In rats, in vitro stimulation of these microglial cells with lipopolysaccharide results in the synthesis of 1,25D [Reference Neveu14].
We accept that there are certain limitations to our study. Given that it was a retrospective study we could not obtain information on vitamin D status or factors that might have influenced serum vitamin D levels (i.e. diet, body mass index, comorbidity, socioeconomic status, personal exposure to sunlight). Intensified transmission of disease during winter periods owing to overcrowded and poorly ventilated housing conditions, as mentioned before, cannot be ruled out as a confounder in this association. Therefore, the involvement of vitamin D in the association of the incidence rate of TBM with sunshine hours is only speculative.
However, an association between sunshine hours and TBM incidence rate has been described for the first time in the present study. This association supports the hypothesis that vitamin D might play a role in the pathophysiology of TBM. Further, prospective studies in which vitamin D status is measured are necessary to determine causality.
ACKNOWLEDGEMENTS
We gratefully acknowledge the statistical assistance of Dr D. J. Kuik. We also thank the South African Weather Service for providing the data on UVB and sunshine hours.
DECLARATION OF INTEREST
None.




