INTRODUCTION
Rotavirus remains the leading cause of childhood diarrhoea worldwide. About 40% of the hospitalizations for childhood diarrhoea worldwide are attributable to rotavirus and more than 0·6 million children aged <5 years die every year from rotavirus infection, mainly in developing countries [Reference Parashar1]. Rotavirus diarrhoea peaks in winter and is rarely identified in summer in temperate regions, for example, the United States [Reference Glass2], Japan [Reference Konno3, Reference Nakagomi4], northern Asian regions [Reference Bresee5], temperate regions in Australia [Reference Bishop6] and Europe [Reference Cook7]. By contrast, it is detected all year round with less obvious seasonality in the tropics, for example, in Bangladesh [Reference Stoll8], southern Asian regions [Reference Bresee5], Bahrain [Reference Dutta9] and Costa Rica [Reference Hieber10]. A study using data from 34 studies from 23 countries on six continents showed that the seasonality of rotavirus diarrhoea is less distinct in the areas within 10° of latitude from the equator [Reference Cook7].
Attempts to explain these findings have been made by relating disease incidence to climate factors such as temperature, humidity and rainfall, but no clear conclusions could be drawn. In Japan, Konno et al. [Reference Konno3] reported that rotavirus infection was associated with lower temperature, but not with relative humidity. In the United States, rotavirus hospitalizations were more common after cold or dry (small amount of rainfall) months than after warm or wet months [Reference Brandt11]. Indoor crowding and low indoor relative humidity in the cold months was suggested to promote transmission of rotavirus [Reference Brandt11]. However, these studies inferred this relation from the increased number of rotavirus infections in cold or dry months, and it may be a consequence of other environmental or behavioural factors that are more (or less) often observed in the cold or dry months. Potential mutual confounding between weather factors (e.g. temperature and humidity) has also not been considered. In Bangladesh, high river level has been suggested as an explanation for a seasonal peak in rotavirus infections during the monsoon [Reference Rahman12].
In this study, we investigated the relationship between short-term variations in ambient temperature, relative humidity and river level and the number of hospital visits owing to rotavirus infections in Dhaka, Bangladesh, using time-series methodology controlling for mutual confounding and for seasonal patterns of unknown cause.
METHODS
The primary outcome in this study is the weekly number of patients with rotavirus diarrhoea visiting the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B) Dhaka Hospital. The ICDDR,B Hospital serves an urban population of about 10 million individuals and provides free treatment to more than 100 000 patients with diarrhoea each year. Every 50th patient attending the hospital for treatment of diarrhoea was enrolled in the surveillance system since 1996. For all patients enrolled in surveillance, faecal samples were collected and tested for rotavirus antigen by an enzyme-linked immunosorbent assay modelled after the Dakopatts commercial kit (Dakopatts, Copenhagen, Denmark). We abstracted individual information on the date of hospital visit and the pathogens identified from each stool specimen during a 6-year period (January 1996 to December 2001). A patient was classified as having rotavirus diarrhoea when rotavirus antigen was identified in the stool specimen. When V. cholerae was identified at the same time, the case was excluded from the analysis (n=199, 6% of all rotavirus-positive patients).
Daily maximum and minimum temperatures and relative humidity in Dhaka were obtained from the Bangladesh Meteorological Department. Daily average temperatures were computed as the mean of the daily maximum and minimum values. The daily river level of the Brigonga River at Mill Barrack in Dhaka was recorded by the Bangladesh Water Development Board. The weekly means for average temperature, relative humidity and river level were calculated from the daily records.
Statistical analysis
We examined the relationships between the weekly number of rotavirus diarrhoea cases and temperature, relative humidity and river level, using generalized linear Poisson regression models allowing for overdispersion. To account for seasonal patterns of rotavirus diarrhoea not directly due to these weather factors, Fourier terms with annual periodicity, up to the sixth harmonic, were introduced into the model. These are sine-cosine pairs, which together can fit any smooth pattern repeated each year [Reference Stolwijk, Straatman and Zielhuis13]. Indicator variables for the years of the study were incorporated into the model to allow for long-term trends and other variations between years. Having adjusted for these seasonal patterns and between-year variations, the short-term associations between climate variables and the number of rotavirus diarrhoea cases can be investigated. An indicator variable for public holidays was incorporated into the model to account for an artificial drop in hospital visits during these weeks. Rainfall was also initially considered, but as there was no evidence that it was associated with the number of rotavirus diarrhoea cases (P>0·1), it was not included in the final analysis. To allow for autocorrelations, an autoregressive term at order 1 was incorporated into the models [Reference Brumback14].
We considered lags (delays in effect) of up to 4 weeks from our previous study [Reference Hashizume15] and other existing literature [Reference Checkley16, Reference D'Souza17]. In initial analyses we fitted natural cubic splines (3 d.f.) [Reference Durrleman and Simon18] to means over lags of 0–4 weeks for (a) mean temperature (b) relative humidity and (c) river level. All three splines were included simultaneously in the model to control for potential mutual confounding.
In summary, the model took the following form:
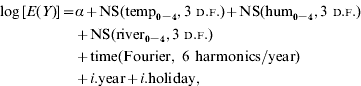
where E(Y) is the expected weekly case count, ‘temp’, ‘hum’ and ‘river’ indicate average weekly temperature, relative humidity and river level, respectively. NS indicates a natural cubic spline function, Fourier represents Fourier (trigonometric) terms, i.year represents indicator variables of year and i.holiday represents an indicator variable for public holidays.
As the plots of the smoothed relationships between temperature or river level and the number of rotavirus cases suggested log-linear associations above a threshold, we then fitted linear threshold models, i.e. models that assume a log-linear increase in risk above a threshold and no increase in risk below the threshold [Reference Armstrong19]. The choice of threshold was based on maximum-likelihood estimation for the temperature or river level over a grid of all possible integer values within a range indicated on the weather–rotavirus diarrhoea graphs. Likelihood profile confidence intervals (CIs) for each threshold were calculated as the thresholds for which deviance of the model was 3·84 more than the minimum. Because the plots of the smoothed relationships with humidity suggested a broadly linear negative relationship, we then fitted a linear model to estimate the effect (slope) [Reference Armstrong19]. With the simple linear model we then examined lag effects in more detail, by fitting linear unconstrained distributed lag models, comprising terms for humidity, temperature and river level at each lag from 0 to 4 weeks [Reference Armstrong19]. The simple linear-threshold model also allowed investigation of the modification of the effect of temperature, humidity and river level between seasons by including interaction terms in the models. The likelihood ratio test was used to test the evidence for the interaction. The season was categorized as summer (March–May), monsoon (June–September) or winter (October–February).
In order to investigate whether the results were sensitive to the levels of control for seasonal patterns, the analyses were repeated using Fourier terms up to the 3rd and 12th harmonics per year. All statistical analyses were carried out using stata 9.0 (Stata Corporation, College Station, TX, USA).
RESULTS
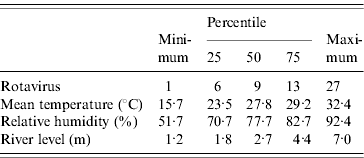
Out of 13 983 hospital visits (one in every 50) for all cause diarrhoea from 1996 to 2001, there were 3115 rotavirus diarrhoea cases, of which 62·5% were <1-year-old and 26·4% were aged between 1 and 2 years. Males (60·7%) were more numerous than females (39·3%). Descriptive statistics for the number of rotavirus diarrhoea cases and meteorological and river-level data are displayed in the Table. The subtropical monsoon climate is clear from the moderately warm temperatures, and high humidity. Temperatures rose rapidly in March, and the average mean temperature was about 29°C during the summer and monsoon seasons (April–September) (Fig. 1). Relative humidity was lowest in March, and increased towards the monsoon season. Rotavirus diarrhoea was common in winter with a lower peak in the middle of the monsoon. There was thus little obvious tracking of seasonal patterns of rotavirus diarrhoea incidence with humidity or temperature. River level peaked in the late monsoon season. River-level data are missing from April 1997 to March 1998.

Fig. 1. Seasonal variations of (a) number of rotavirus diarrhoea patients, (b) relative humidity (RH), (c) temperature and (d) river-level data in Dhaka, 1996–2001.
Table. Distribution of the weekly numbers of rotavirus diarrhoea cases in the ICDDR,B hospital, and meteorological data in Dhaka, 1996–2001
Relationship with temperature
The relationships between the relative risk of hospital visits for rotavirus diarrhoea and temperature are shown in Figure 2. In the crude relationship, the potential risk of rotavirus diarrhoea decreased as temperature increased from the coldest temperatures. The risk began to rise as the temperature increased above ~28°C, producing a ⋃-shaped relation. However, there was no evidence for increased risk at low temperatures after adjustment for humidity, river level, seasonal patterns, between-year variations and public holidays, while an increase in risk at the highest temperatures remained. The estimated threshold was 29°C (95% CI 28–30). For a 1°C increase above the threshold, the number of rotavirus diarrhoea cases increased by 40·2% (95% CI 19·4–64·6). The effect of high temperature was significant at lags 0 and 3 in the distributed lag model, while little effect was observed for any of the other lags (Fig. 3).

Fig. 2. Relationship between relative risk (RR) of rotavirus diarrhoea (scaled to the mean weekly number of rotavirus diarrhoea cases) and average temperature over lags of 0–4 weeks (shown as a 3 d.f. natural cubic spline). (a) Crude relationship and (b) relationship adjusted for humidity, river level, seasonal patterns, between-year variations and public holidays. The centre line in each graph shows the estimated spline curve, and the upper and lower lines represent the 95% confidence limits.

Fig. 3. Percent change (and 95% CIs) for high temperature effects of varying periods of risks on rotavirus diarrhoea (unconstrained distributed lag models). Percent change shows the change in the number of the cases for every 1°C increase above the threshold (29°C).
Relationship with humidity
The relationships between the relative risk of hospital visits for rotavirus diarrhoea and humidity are shown in Figure 4. In the crude relationship, there was no obvious pattern observed. After adjusting for temperature, seasonal patterns, between-year variations and public holidays, the pattern shows a linear negative slope with humidity, which was confirmed by fitting a linear model (P=0·05 by Wald test). For a 1% decrease in humidity, the number of rotavirus diarrhoea cases increased by 2·6% (95% CI 0·0–5·3). The distributed lag model showed that there was suggestive evidence for an effect of humidity at lag 0, and this effect decreased to null thereafter (Fig. 5).

Fig. 4. Relationship between relative risk (RR) of rotavirus diarrhoea (scaled to the mean weekly number of rotavirus diarrhoea) and average humidity over lags of 0–4 weeks (shown as a 3 d.f. natural cubic spline). (a) Crude relationship and (b) relationship adjusted for temperature, river level, seasonal patterns, between-year variations and public holidays. The centre line in each graph shows the estimated spline curve, and the upper and lower lines represent the 95% confidence limits.

Fig. 5. Percent change (and 95% CIs) for humidity effects of varying periods of risks on rotavirus diarrhoea (unconstrained distributed lag models). Percent change shows the change in the number of the cases for every 1% decrease in relative humidity.
Relationship with river level
The relationship between the relative risk of hospital visits for rotavirus diarrhoea and river level is shown in Figure 6. In the crude relationship, there appeared to be an increase in the number of cases of rotavirus diarrhoea when the river level was higher than 4–5 m. This pattern was similar (with slight loss of precision) after adjusting for temperature, relative humidity, seasonal patterns, between-year variations and public holidays. The estimated threshold was 4·8 m (95% CI 4·2–5·1). For each 10-cm increase above the threshold, the number of hospital visits for rotavirus diarrhoea increased by 5·5% (95% CI 3·2–7·8). The distributed lag model showed that there was suggestive evidence for an effect of river level at lag 1, while little effect was observed for any of the other lags (Fig. 7).

Fig. 6. Relationship between relative risk (RR) of rotavirus diarrhoea (scaled against the mean weekly number of rotavirus diarrhoea) and average river level over lags of 0–4 weeks (shown as a 3 d.f. natural cubic spline). (a) Crude relationship and (b) relationship adjusted for humidity, temperature, seasonal patterns, between-year variations and public holidays. The centre line in each graph shows the estimated spline curve, and the upper and lower lines represent the 95% confidence limits.

Fig. 7. Percent change (and 95% CIs) for high river-level effects of varying periods of risks on rotavirus diarrhoea (unconstrained distributed lag models). Percent change shows the change in the number of the cases for every 10 cm increase above the threshold (4·8 m).
The effect of temperature on rotavirus infections differed across seasons (P=0·02), while the effects of humidity and river level did not. When in sensitivity analyses the degree of control for seasonal patterns was halved (three harmonics) or doubled (12 harmonics), the estimates of the effect of temperature, humidity and river level changed little (results not shown).
DISCUSSION
In Bangladesh, there appears to be a significant positive association between the number of hospital visits for rotavirus diarrhoea and temperature above a threshold after adjusting for potential confounding by humidity, river level, seasonal patterns, between-year variations and public holidays. An inverse association between relative humidity and the number of rotavirus infections was also observed. Higher river level was associated with increased risk of rotavirus infections independently of these weather factors.
There have been many reports that have attempted to identify an association between the incidence of rotavirus infections and weather factors. In temperate regions, a peak in rotavirus infections is observed in the colder and drier months of the year [Reference Ansari, Springthorpe and Sattar20]. In tropical and subtropical settings as well, the peak of rotavirus infections occurs in cool dry seasons, although the seasonality is less obvious in some developing countries. Therefore, lower air temperature, especially in combination with a dry atmosphere, has been suggested to increase the number of rotavirus infections [Reference Ansari, Springthorpe and Sattar20]. However, previous studies did not take into account potential confounding due to seasonally varying factors other than weather factors, or mutual confounding between weather factors, which are likely to vary between regions. For example, Konno et al. [Reference Konno3] reported that there was a higher number of rotavirus diarrhoea cases in the months of low temperature in winter, but no association was found with relative humidity in Yamagata, Japan. The discrepancy in the results of association with low temperature could be due to the effect of seasonally varying factors, which was controlled in the current study but not in previous studies. The possibility that previous observations may be confounded is supported by the results in this study unadjusted for seasonal patterns, which found a significant negative association between temperature and the number of cases. Similarly, in the unadjusted analysis, no association was found between humidity and the number of the cases, but a negative association appeared after adjusting for seasonal patterns.
The seasonal pattern of rotavirus diarrhoea cases may be a consequence of unmeasured factors apart from weather factors. These factors include seasonal patterns linked to human activity and environmental factors other than weather. In Washington, DC, more children were hospitalized for rotavirus gastroenteritis after a month of cold or dry weather than after a matched calendar month of warm or wet weather [Reference Brandt11]. In that study, temperature and humidity was not mutually adjusted, and this could also be a reason for the discrepancy between the results in Washington and those of this study.
The absence of evidence in this study for a link between low temperature and increasing number of rotavirus diarrhoea cases contrasts with findings from some laboratory studies. The survival of human rotavirus in faeces was longer under lower temperatures (range 4–37°C) than higher temperatures, in a laboratory setting [Reference Moe and Shirley21]. The survival of bovine rotavirus in water droplets in the air was also longer at lower temperatures, regardless of relative humidity [Reference Moe and Harper22]. These studies, however, were conducted under laboratory settings and hence the results may not be applicable to the real environment. The biological plausibility of a potential high temperature effect and its lag structure (significant at lags 0 and 3 weeks) is not clear. As the most high-temperature weeks are concentrated during the monsoon season, and as we obtained evidence for a modification of temperature effects across seasons in this study, the effect of temperature may be exerted through complex temperature-dependent pathways in the monsoon season. Further research using the same approach in different climate conditions, for example, in temperate regions, could contribute deeper insight into the effects of temperature.
The finding of a negative association between cases of rotavirus diarrhoea and humidity is more consistent with laboratory evidence. Relative humidity may affect the survival of rotaviruses in air and on fomites and environmental surfaces. At low or medium relative humidity (13–55%), human rotavirus can remain viable on plastic, glass and stainless steel for more than 10 days at room temperature [Reference Moe and Shirley21, Reference Sattar23]. Raising the relative humidity to 80% results in a rapid loss of infectivity [Reference Sattar23]. Survival of human rotavirus in air at 20°C was best at 50% relative humidity, with a half-life of 44 h; at low (30%) and high (80%) relative humidity, the half-life was decreased to 24·4 h and 3·8 h, respectively [Reference Ijaz24]. Thus, in the range of relative humidity in this study (52–92%), the survival of human rotavirus increased with lower relative humidity in the fixed temperature in the laboratory setting. Dry conditions would also tend to encourage the formation of virus-laden dust and droplet nuclei [Reference Brandt25]. However, no direct evidence shows the spread of rotavirus gastroenteritis through these pathways, and it is not clear whether the ability of rotavirus to survive in the environment is the basis for the inverse association between relative humidity and the number of rotavirus infections found in this study. The short lag of the effect of relative humidity (lag 0) is broadly in accordance with the short incubation period of rotavirus (~24–72 h) [Reference Chin26] even taking into account the lag owing to visiting the hospital after symptoms appeared (73% of rotavirus patients visited the hospital within 3 days after symptoms appeared).
The independent effect of high river level on the increased risk of rotavirus infections could be due to exposure to flood water. In Bangladesh, post-flood increases in rotavirus diarrhoea have been reported [Reference Ahmed27, Reference Fun28]. Floods adversely affect water sources and supply systems as well as sewerage and waste disposal systems [Reference Parker and Thompson29]. Sewage, which is known to contain large numbers of infectious rotavirus particles [Reference Smith and Gerba30], may be disposed of in raw form and infectious rotaviruses can be recovered from sewage-polluted surface water [Reference Hejkal, Smith and Gerba31]. There have also been reports of outbreaks of waterborne rotavirus gastroenteritis [Reference Ansari, Springthorpe and Sattar20]. For example, river water used for bathing and for washing utensils was suspected of being involved in virus spread in the outbreak of rotavirus gastroenteritis in Brazil [Reference Linhares32]. A faecally contaminated water supply was the primary vehicle in an outbreak of rotavirus gastroenteritis with 12 000 cases in China [Reference Hung33]. Survival of rotavirus in water has also been investigated. Simian rotavirus SA-11 was shown to survive in experimentally contaminated fresh and estuarine waters for several days at 20°C, especially in water heavily polluted with sewage [Reference Hurst and Gerba34]. Studies with rotavirus isolated from humans showed survival for several weeks at either 20°C or 4°C in river water [Reference Raphael, Sattar and Springthorpe35]. Infectious particles of rotavirus have been recovered from a variety of natural waters including rivers, lakes and groundwater [Reference Deetz36]. The results of this study support the idea that water or factors associated with river level could have been a frequent transmission route of rotavirus in Bangladesh.
Clarifying the effects of climate on the spread of rotavirus diarrhoea could contribute to improvements in the precision of the WHOs estimates for diarrhoeal disease burden attributable to global climate change [37], which did not consider the relative contribution of different pathogens to overall diarrhoea incidence despite the fact that their climate sensitivities are likely to be different. Because the proportion of rotavirus diarrhoea among all hospitalized diarrhoea varies from 24% to 45% between world regions [Reference Bresee5, Reference Cunliffe38–40], the results could also improve estimates of regional health risks due to diarrhoeal disease associated with global climate change.
A strength of this dataset is the relative completeness of coverage of the population in Dhaka. The hospital serves an urban population of about 10 million individuals and provides free treatment to more than 100 000 cases of diarrhoea each year. Therefore, the data used in this study are reasonably representative of the underlying severe diarrhoeal morbidity of the population in Dhaka. Less severe cases would be less likely to be included, but this does not pose a threat to the validity of the comparisons over time, which is the subject of this study.
However, this study is not free from biases. Although cases excluded because the stool sample also included V. cholerae were most likely to have attended the hospital because of the cholera, those that would have attended in the absence of cholera should have been included. It was impossible to identify these cases, however, and the total of such comorbidity was small. There is also the possibility of residual confounding, although the robustness of the results to varying degrees of seasonal control was reassuring.
There are important uncertainties related to the extrapolation of the relationships in this study to others with different climate and geography. In particular, the observed association between river level and the number of rotavirus infections may be specific to places characterized by low land with bodies of water that are vulnerable to flooding. The association may also be greatly dependent on the degree of hygiene and sanitation in an area. Therefore, these findings may not pertain to other places.
In this study, weather factors were found to explain departures of the number of rotavirus diarrhoea cases from the usual seasonal pattern. This does not mean that these factors can explain the usual seasonal patterns themselves. The transmission of rotavirus is complex and multifactorial, involving both host and environmental factors. Some of these environmental factors have not been considered in this study, which suggests that temperature, relative humidity and river level might be important environmental determinants of rotavirus infectivity. Further work to clarify the role of weather in the seasonality of rotavirus infections in Bangladesh and elsewhere would be of interest.
ACKNOWLEDGEMENTS
We gratefully acknowledge the support of the ICDDR,B. This study was supported by Daiwa Foundation Small Grants. M. H. was supported by the Foundation for Advanced Studies on International Development and GlaxoSmithKline.
DECLARATION OF INTEREST
None.










