INTRODUCTION
Staphylococcus aureus are ubiquitous bacteria that can cause severe disease in children and adults. One way of transmission of this organism is via carriers (individuals who are asymptomatically colonized with an organism). Although colonization itself does no harm to the host, it is a known risk factor for developing and transmitting clinically relevant infections [Reference Wertheim1].
Increased risk of S. aureus carriage and infection with methicillin-resistant S. aureus (MRSA) is associated with many occupations, including healthcare workers, military personnel, agricultural workers, and veterinary practitioners [Reference Johnston2–Reference Aiello6]. Despite the fact that childcare employees are at risk of many common infections and outbreaks of MRSA in childcare centres have been reported, data on S. aureus carriage in this occupational group is limited [Reference Adler7–Reference Jensen14]. In studies published since January 2000, carriage of all S. aureus was reported to be between 14·3% and 31·6% in childcare workers [Reference Rosen and Ryan15–Reference Sesli17], while MRSA was found in 3·1% of staff working at a childcare facility in Texas [Reference Hewlett18]. To our knowledge, only one study has provided data on the risk of S. aureus carriage in childcare workers compared to the general population. Rosen & Ryan [Reference Rosen and Ryan15] reported that the S. aureus carriage rate in childcare employees was 14·29%, while the rate in adults not employed at a childcare facility was 10·77%. However, this study was relatively small and did not provide data on potential confounders.
We sought to (1) determine if childcare employees are at increased risk of S. aureus colonization compared to individuals not employed in a childcare centre and (2) to identify predictors of S. aureus carriage in childcare employees.
MATERIALS AND METHODS
Selection of childcare centers
We conducted a cross-sectional study of S. aureus in 12 childcare facilities located in Iowa. All protocols were approved by the University of Iowa Institutional Review Board. Childcare facilities were identified from the Johnson County list of registered and licensed facilities, as well as through personal connections [19]. Contact letters were sent out to all facilities on this list. Follow-up calls were conducted, with priority given to larger licensed facilities, starting at the top of an alphabetized list of facilities. Facilities were scheduled for visits until the desired sample size (110 employees) was reached. All centre visits occurred between February 2009 and February 2010.
Recruitment of participants
Sample sizes of employees and unexposed adult groups were calculated assuming a 50% carriage rate in employees and a 30% carriage rate in unexposed adults at alpha = 0·05 and beta = 0·80. Adult participants were aged ⩾18 years (or able to obtain parental consent) and children sampled were aged >6 months. After the director gave permission for the study team to recruit at the centre, employees and parents of children at a facility were recruited with fliers 1 week prior to the site visit. Unexposed adults who were not employed in childcare facilities were matched on age and gender frequencies, and were recruited through flier postings and mass emails sent to the University of Iowa College of Public Health and undergraduate populations during December 2009. Informed consent documents were signed by all adult participants and at least one parent of child attendees.
Questionnaire data
Questionnaires were administered to employee and unexposed adult participants, as well as to one parent of child participants. A facility questionnaire, collecting data on facility characteristics and sanitation practices, was completed by the director of each centre.
Swabbing of participants
All samples were collected using cotton-tipped transport swabs (BD BBL Culture Swabs with Liquid Stuart media, Becton, Dickinson and Company, USA and Remel, USA) as previously described [Reference Smith4]. Nasal and throat swabs were collected from adults, while only nasal swabs were collected from children in order to minimize discomfort. All samples were stored in Liquid Stuart medium following collection, kept at 4 °C during transport, and processed within 24 h of collection.
S. aureus isolation and confirmation
S. aureus and MRSA were isolated and confirmed as previously described [Reference Smith4]. Briefly, swabs were inoculated into S. aureus enrichment broth and subsequently plated onto Columbia colistin-nalidixic agar (CNA) with 5% sheep blood (Columbia CNA, Remel) and selective MRSA agar plates (BBL CHROMagar MRSA, Becton, Dickinson and Company) for identification. S. aureus isolates were confirmed using Gram stain, the catalase test, the slide coagulase test and a S. aureus latex agglutination assay (Pastorex Staph-plus, Bio-Rad, France). Methicillin resistance was identified with a MRSA latex agglutination test (Oxoid Ltd, UK) and confirmed using a mecA PCR [Reference Oberdorfer20]. A participant was considered colonized if either a nose swab, a throat swab, or both yielded S. aureus.
Statistical analysis
Statistical analysis was performed using SAS 9.2 (SAS Institute Inc., USA). Prevalence of all S. aureus and MRSA in employees, children, and unexposed adults was calculated. For data collected through questionnaires, frequencies were calculated for categorical variables, as well means, standard deviations, minimums, and maximums for continuous variables. Univariate and multivariate analyses were conducted on data from (1) all adults to determine risk of carriage in employees compared to an unexposed population; (2) children to identify variables that would be used as potential predictors for employees; and (3) childcare employees to determine if variables associated with their work predict carriage.
Univariate analysis was performed separately using carriage of any S. aureus and MRSA as outcomes. χ2 or Fisher's exact test were used to identify potential predictors for the multivariate analysis. In order to maintain a parsimonious model, only variables that were significantly associated at P < 0·15 with both the exposure and outcome were considered for multivariate modelling. Multivariate analysis was performed with unconditional logistic regression due to the fact that age and gender matching was not one-to-one. Models were created using generalized estimating equations to adjust for clustering among facilities. Due to small cell counts, exact logistic regression was used for multivariate modelling of MRSA carriage in employees. Model selection was conducted using manual backwards elimination.
RESULTS
Centre characteristics
Letters explaining the study were sent to about 280 licensed and registered facilities in Johnson County listed on the Iowa Department of Health and Human Services website. Study personnel were able to speak with 25 directors, 13 of whom agreed that their facility would participate. Data and samples from one centre were discarded due to fungal overgrowth of cultures, and one centre did not have to participate because our designated sample size was achieved. This study contains data from 11 facilities that were visited between February 2009 and February 2010. Capacities for children, as reported by the facility director, ranged from 16 to 168 (mean capacity 53 children, median capacity 33 children), while capacities for employees ranged from three to 60 employees (mean capacity 21 employees, median 18 employees).
Employee characteristics
A total of 110 employees participated in this study. Employee participation rates at each centre ranged from 35·0% to 100·0%, and the average participation rate was 59·3%. The mean age of employee participants was 29·7 years (median age 24 years, range 16–64 years); 92·6% of the employee population was female. Carriage of any S. aureus (MSSA or MRSA) in employees was 34·5% (16 carried S. aureus in the nose only, 12 carried it in the throat only, and 10 carried it in both sites). The carriage rate per facility ranged from 11·8% to 66·7%. Carriage of MRSA in employees was 3·63% (three carried MRSA in the nose only and one in the throat only). The prevalence in each facility ranged from 0% to 16·7% (Supplementary Table S1).
Child characteristics
Eighty-one children participated in this study (mean child participation rate was 22·8%, range 5·0–68·8%). The average age of child participants was 2·97 years (median age 3 years, range 6 months to 7 years), and females accounted for 57·9% of the child participants. Prevalence of S. aureus in children was 19·8%, while 1·23% (one child) carried MRSA. Prevalence in children at each facility ranged from 0% to 42·9%, although the only child who participated at one facility was colonized, yielding a carriage rate of 100% (Supplementary Table S1).
Unexposed adult characteristics
There were 111 adults not employed at a childcare facility that participated in this study. The average age of unexposed adult participants was 31·8 years (median age 27 years, range 18–78 years). Females made up 88·3% of the unexposed population. The prevalence of S. aureus in unexposed adults was 33·3% (13 nose only participants, 11 throat only participants, and 13 carried S. aureus in both sites), while MRSA was carried by one (0·90%) unexposed adult, who was colonized only in the throat.
Analysis of exposed and unexposed adults
The crude odds ratio (OR) for childcare employment as a risk for any S. aureus colonization was 1·09 [95% confidence interval (CI) 0·62–1·90, P=0·77], while the crude OR for MRSA colonization was 4·23 (95% CI 0·47–38·48, P = 0·21) (Table 1). Variables that were significantly associated with the outcome (S. aureus carriage) in all adults at P < 0·15 are shown in Table 1. Variables that were significantly associated with the exposure (employment at a childcare facility) are shown in Table 2.
Table 1. Questionnaire variables and their association with colonization (P < 0·15) with any S. aureus in the adult study population

OR, Odds ratio; CI, confidence interval.
* Based on Cochran–Armitage test for trend.
† Autumn/winter season included October–March; spring/summer included April–September.
‡ Categorical/ordinal variable – individual odds ratios not calculated.
Table 2. Questionnaire variables whose frequencies differed (P < 0·15) between childcare employees and unexposed individuals

OR, Odds ratio; CI, confidence interval; URTI, upper respiratory tract infection; SSTI, skin and soft tissue infection.
* Based on Cochran–Armitage test for trend.
† Autumn/winter season included October–March; spring/summer included April–September.
‡ Categorical/ordinal variable – individual odds ratios not calculated.
To adjust for confounders, multivariate analysis was performed using variables that were associated with both the exposure and the outcome at P < 0·15, which were season, age, a household contact who developed an influenza-like illness in the past 12 months, and a household contact with exposure to cattle. The adjusted OR for S. aureus carriage in childcare employees was 0·68 (95% CI 0·31–1·50, P=0·34) (Table 3). The OR for MRSA carriage was 3·28 after adjusting for a history of cigarette smoking and age of the oldest child in the household (95% CI 0·31–169·02, P = 0·53) (data not shown).
Table 3. Final multivariate model of risk of S. aureus using generalized estimating equations to adjust for clustering*
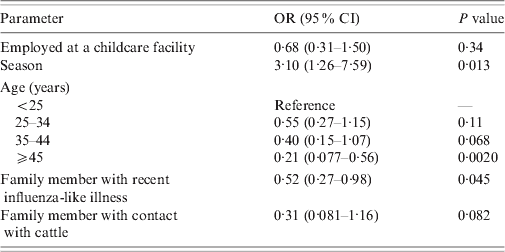
OR, Odds ratio; CI, confidence interval.
* Model-based standard error estimates.
Analysis of children
Variables that predicted S. aureus carriage in children at P < 0·05 included asthma, history of an acute asthma attack, the number of times the parent exercised at a gym in the past month, and recent contact with animals, goats, and sheep. The proportion of child participants reporting these variables in each facility was considered as a potential predictor in the multivariate analysis of risk factors in employees.
Analysis of childcare employees
Potential risk factors for carriage of S. aureus that were statistically significant during univariate analysis at P < 0·15 were considered for multivariate analysis. None of the individual-level characteristics of employees (e.g. age of children supervised or type of duties performed) was a significant predictor of S. aureus carriage (Supplementary Table S2). Due to the high number of facility-level variables (such as years the centre had been in operation and hand washing practices as reported by the director) that were statistically significant during univariate analysis, final variables for entering the multivariate model were chosen according to a lowered P value of 0·05, biological plausibility, and presence of collinearity with another variable (Supplementary Table S3). The variables ‘centre separates children at least once during the day’, ‘children wash hands upon arrival at the centre’, and ‘child participants reporting contact with animals’ were chosen for multivariate consideration. In the final multivariate model, ‘children wash hands upon arrival at the centre’ was significant at P < 0·0001 (OR 0·17, 95% CI 0·095–0·32) (Table 4).
Table 4. Final multivariate model of risk of S. aureus carriage in childcare employees using generalized estimating equations to adjust for clustering*

OR, Odds ratio; CI, confidence interval.
* Model-based standard error estimates.
DISCUSSION
We did not find a significant increased risk of S. aureus colonization in childcare employees. In this study, washing children's hands upon their arrival at childcare facilities decreased the odds of S. aureus carriage in employees by 83%.
This study had several important limitations. Due to the recruitment methods used for the unexposed group, we were unable to calculate participation rates in the control group. Our unexposed group differed from the childcare employees in several variables (Table 2). In addition, we recruited many unexposed individuals from the University of Iowa College of Public Health; these individuals may be more likely to carry S. aureus than the general population, pushing the effect of childcare exposure towards the null.
Participation rates and sample sizes for employees and children were low. Due to the fact that we visited a centre at most twice, we were unable to access all different shifts of employees, which may have contributed to the low participation rate. Participation rates in children were likewise low, even when the majority of children were present at the centre at the time of sampling. Childcare facility recruitment visits were conducted during the 2009 H1N1 outbreak and parents may have mistakenly assumed a more invasive sampling procedure was required, contributing to the low participation rate. The timing of sampling during the H1N1 outbreak may also have led to an increased focus on prophylactic measures to prevent transmission within facilities, leading us to underestimate risk of S. aureus in childcare employees.
The number of MRSA isolates in this study was too low to provide meaningful analyses and conclusions. Because of the limitations listed above, more extensive studies with larger sample sizes conducted outside of influenza season are needed to obtain a more accurate picture of prevalence and risk factors for S. aureus and MRSA carriage in childcare workers.
The external validity of our results might also be affected by the type of facilities we visited. We had no means of collecting data on facilities that refused to participate, leading to the potential for unrecognized selection bias. It is possible that facilities that were not stringent in following hygiene standards were also less likely to participate, leading to a ‘clean facility bias’. The majority (11/12) of facilities participating in this study were licensed facilities, which tend to be larger and are held to more stringent standards than non-licensed facilities [21]. Our results, therefore, may not be generalizable to smaller or in-home centres. We suspect that due to little oversight of sanitation practices and the possible presence of pets, employees at non-licensed facilities may be at greater risk of S. aureus carriage than the participants of this study.
We did not identify any individual-level predictors of carriage within the childcare employee population. Children washing their hands upon arrival at the facility, on the other hand, predicted a decreased risk of S. aureus carriage in employees. Given the apparent highly significant protective association of this variable on risk of carriage in employees, it may be prudent to recommend this practice as standard protocol in childcare facilities.
The results of analyses of facility-level risk factors in employees should be viewed with caution. Because many variables were related to the facility and applicable to all employees at that facility (for example, the number of years the centre had been in operation), the number of variables displaying collinear relationships was high. While every effort was made to narrow variables down to those with biological plausibility, it is possible that the effects of another variable could be masking the true relationship between hand washing upon arrival and carriage in employees. The potential decrease in risk of S. aureus carriage in childcare employees as a result of washing children's hands upon arrival would best be studied using a randomized controlled hand washing intervention trial. However, recommending hand washing upon arrival is unlikely to have adverse effects, even if the relationship has not been accurately characterized.
While we did not find increased odds of S. aureus colonization in childcare employees, more extensive, prospective studies are needed to further characterize this risk and any health consequences that may affect this rarely studied occupational group. Recommending that children wash their hands upon arrival at childcare centres may be prudent, not only in the context of S. aureus carriage, but for reduction of pathogen transmission in general.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0950268812002415.
ACKNOWLEDGEMENTS
The authors contributed equally to this work. This project was funded by the National Institute for Occupational Safety and Health through a small project grant provided by the Heartland Center for Occupational Health and Safety and by general funds through the Center for Emerging Infectious Diseases at the University of Iowa. Fellowship support was provided by the Occupational Epidemiology Training Program within the National Institute for Occupational Safety and Health-funded Heartland Center for Occupational Safety and Health (T42 OH008491-03). We thank Jennifer Kroeger, Anne Dressler, Shylo Wardyn, and Blake Hansen for technical assistance.
DECLARATION OF INTEREST
None.






