Introduction
Patients with end-stage renal disease (ESRD) including those receiving dialysis treatment and recipients of kidney transplants are known to be at a high risk for the development of bloodstream infections (BSI) [Reference Dalgaard1–Reference Nielsen3]. However, it is not well defined whether renal dysfunction in the absence of ESRD increases the risk for BSI. James et al. conducted a large cohort study of adults aged >65 years in Calgary, Canada during 2001 and 2004 and observed that patients with estimated glomerular filtration rates (eGFR) of 45–59, 30–44 and <30 ml/min/1.73 m2 had stepwise increased hazard ratios for BSI of 1.24, 1.59 and 3.53, respectively [Reference James4]. A number of other studies have investigated links between renal dysfunction and infection risk in other cohorts [Reference James4–Reference Wang7].
Population-based studies that include all episodes of disease occurring among residents of a defined region are optimal designs for defining the epidemiology of an infectious disease. While there are population-based studies that have examined the risk for the development of BSI related to ESRD, to our knowledge, no previous studies have examined renal dysfunction risk for BSI in non-selected populations [Reference Dalgaard1–Reference Nielsen3]. The objective of this study was to quantify the risk for the development of and mortality from community-onset BSI associated with varying levels of renal function among residents of the western interior of British Columbia, Canada.
Methods
Study population
Population-based surveillance was conducted in the western interior of British Columbia, Canada. The study population (2017 population 182 422) included residents of the city of Kamloops and a number of surrounding smaller towns, villages and rural areas within a large geographical area [Reference Laupland8]. All residents 18 years of age and older with incident community-onset BSIs between April 2010 and March 2017 were included. The Interior Health Research Ethics Board approved this study (File 2013-14-052-I).
Data
Detailed demographic (age, gender), clinical (co-morbid conditions, infection focus), infecting organisms, admission to hospital and 30-day all-cause case-fatality rates were obtained by case-by-case review as per previously described [Reference Laupland9, Reference Dagasso10]. Co-morbid illnesses were classified using the definitions of Charlson [Reference Charlson11]. Patients with community-onset BSIs were further subclassified as healthcare-associated or community-associated as per the definitions of Friedman et al. [Reference Friedman12]. eGFR were obtained from the regional corporate data warehouse for all patients who had at least one sample submitted to a hospital-based laboratory in the region. All determinations within the 90 days preceding the index BSI were included and the highest eGFR was used to establish renal dysfunction grouping using the CKD-EPI equation [Reference Levey13]. These were initially grouped according to eGFRs of ≥90, 60–89, 45–59, 30–44, 15–29 and ⩽15 ml/min/m2, respectively. Where data were unavailable, these were assumed to be ⩾90 ml/min/m2.
Statistical analysis
All analyses were performed using Stata/SE 15.1 (StataCorp, College Station, Texas, USA). Prior to the analysis of continuous variables, the underlying distribution was assessed using histograms. Skewed data were described using the median with interquartile range (IQR) and groups were compared using the K-sample median test. Fisher's exact test was used for categorical data. We a priori specified that each of the individual pre-specified eGFR categories would be grouped into fewer categories in the event of small numbers if not significantly different.
Incidence rate ratios (IRR) with 95% confidence intervals (CI) were calculated to express the risk among residents for the development of a BSI associated with the degrees of renal dysfunction. The number of individuals residing within the region with renal dysfunction were estimated as 7.6%, 0.4% and 0.10% for eGFRs of 30–59, 15–29 and <15 ml/min/1.73 m2 based on prevalence estimates summarized by Hill et al. [Reference Hill14]. Incident rate ratios were then calculated by comparing the incidences of with varying levels of renal dysfunction as compared to a reference category of those with eGFR ≥60 ml/min/1.73 m2.
A logistic regression model was developed to examine independent risk factors for 30-day fatality among patients with first episodes of community-onset BSI. The initial model included the degree of renal dysfunction, age, gender, diabetes mellitus and other variables significant to the P < 0.1 level as identified in univariate analyses. The most parsimonious model was then derived using backward stepwise variable elimination. Eliminated variables were tested by reinsertion into the presumptive model to test significance one-by-one. Model calibration and discrimination were assessed in the final model using the goodness of fit test and the area under the receiver operator characteristic curve. A P-value of <0.05 was considered statistically significant for all analyses.
Results
A total of 1663 episodes of community-onset BSI were identified among residents of the western interior during the study period of which 1597 (96%) were in adults. Forty-four cases occurred among ESRD patients and were further excluded leaving a final cohort of 1553 episodes for analysis.
The median age of the cohort was 68.1 (IQR 55.3–78.5) years, 841 (54%) were male, and most cases (1379; 89%) were admitted to hospital for management. Among these community-onset BSI, 905 (58%) and 648 (42%) were community- and healthcare-associated, respectively. Overall, 612 (39%), 498 (32%), 259 (17%), 142 (9%), 34 (2%) and 8 (1%) had preceding eGFRs of ≥90, 60–89, 45–59, 30–44, 15–29 and <15 ml/min/m2, respectively.
The presence of renal dysfunction was associated with significantly increased risk for the development of community-onset BSI among the western interior population as shown in Table 1. There was a categorical stepwise increase in risk for the development of BSI associated with a decrease in kidney function that was most notable among those with healthcare-associated infection (Table 1).
Table 1. Risk for community-onset bloodstream infections according to the degree of kidney function

eGFR, estimate glomerular filtration rate.
A number of characteristics were investigated as the determinants of community-onset BSI in association with varying levels of kidney function and are shown in Table 2. Although there was a significant proportional variability observed between age and renal function, there was no evident trend across ordered categories (Table 2). Among each of the individual co-morbid illnesses included in the Charlson Comorbidity Index that were evaluated, only hemiplegia, liver disease and HIV infection showed significant proportional variability according to kidney function categories (Table 2; non-significant variables not shown). Diabetes mellitus was present in 247 (22%), 92 (23%) and 14 (33%) episodes associated with eGFRs of ≥60, 30–59 and <30 ml/min/m2 (P = 0.2), respectively.
Table 2. Characteristics of adult patients with episodes of community-onset bloodstream infection according to renal function

eGFR, estimate glomerular filtration rate.
Among the cohort of first presentations with community-onset BSI (n = 1401), crude 30-day case-fatality rates were 14% (77/551), 15% (69/461), 10% (24/234), 17% (20/118), 29% (9/31) and 33% (2/6) with preceding eGFRs of ≥90, 60–89, 45–59, 30–44, 15–29 and <15 ml/min/m2 (P = 0.04), respectively. A multivariable logistic regression model was developed that had good discrimination (area under receiver operator characteristic curve 0.77) and calibration (goodness of fit P = 0.8). As shown in Table 3, after controlling for a number of co-variates, patients with eGFR <30 ml/min/m2 (odds ratio (OR) 2.3, 95% CI 1.0–5.2) but not 30–59 ml/min/m2 (OR 1.0, 95% CI 0.6–1.4) were at increased risk for death.
Table 3. Logistic regression modelling of factors associated with 30-day all-cause case-fatality among patients with first episodes of community-onset bloodstream infection
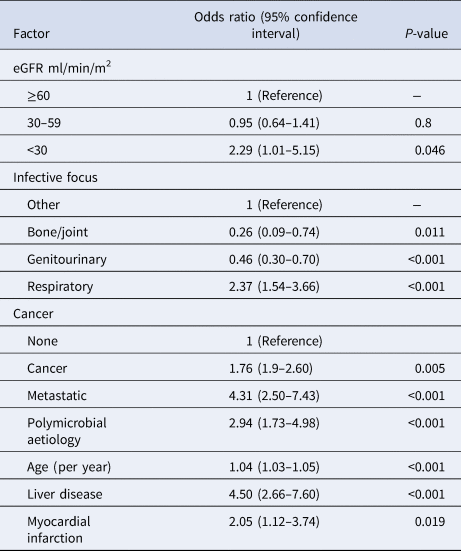
eGFR, estimate glomerular filtration rate.
Discussion
This study is important as it adds to the small body of literature identifying renal dysfunction in the absence of ESRD as a significant risk factor for developing BSI. Additionally, we identified that eGFR <30 is an independent risk factor for mortality from BSI. These data raise the important possibility that renal dysfunction is a modifiable risk factor for serious infection and that enhanced preventative and management efforts may not only lead to a reduction in morbidity and mortality from kidney disease directly but also indirectly through infection prevention.
There are few studies for which to directly compare. Most importantly, James et al. in their study in Calgary found that increasing degrees of renal dysfunction were associated with concomitant increased risk for BSI in subjects aged 65 and older [Reference James4]. They further found an increased risk for death associated with renal dysfunction. Our results are similar and extend findings to a different population in Canada that includes young and middle-aged adults. Other studies have examined renal dysfunction and associated risk with pneumonia, infections in general and community-acquired infections [Reference Dalrymple5, Reference Viasus15–Reference Foley17]. While limited, there is an increasing body of evidence that supports the risk for infection in association with renal dysfunction.
There are a number of limitations that merit discussion. First, although we had a sizable cohort of 1553 cases, we had relatively small numbers of cases in the more advanced degrees of kidney dysfunction such that we may have underpowered to detect significant differences among groups. Second, we based our assessment on determining the best eGFR within 3 months of admission such that the possibility exists that some of the abnormal renal functions may have been a result rather than a contributing factor infection. Third, we did not have linked individual-level data on co-morbidities and renal function among population controls. This precludes multivariable analysis of risk for acquiring BSI. In addition, although the prevalence rates we used are based on an extensive systematic review, they do add a degree of imprecision to our findings. Finally, not all of our subjects had preceding renal function documented and we assumed missing cases to have normal function. Thus, our estimates should be viewed as conservative.
Conclusions
In summary, this study demonstrates that patients who have non-dialysis-dependent renal impairment are at increased risk for the developing and dying from community-onset BSI and that this occurs in an impairment-dependent fashion. Efforts to reduce the occurrence of chronic renal disease may be expected to reduce the major burden associated with these important infections.
Acknowledgements
We thank Ursula Kaeser and Holly Buhler, Interior Health, for providing the kidney function data from the corporate warehouse.
Financial support
This work was supported by the British Columbia Provincial Renal Agency and the Interior Health Renal Program.
Conflict of interest
None.





