INTRODUCTION
Campylobacter is a common cause of acute bacterial gastroenteritis in children worldwide, and the most common cause in many developed countries [1–Reference Bowman, Flint and Pollari4]. However, most children who contact Campylobacter infections are not part of recognized outbreaks and the source of the infection in many sporadic cases remains unknown. Even though a number of epidemiological studies have been conducted to assess the risk factors for sporadic infections in children [Reference Denno5–Reference Tenkate and Stafford11], the international epidemiological data are still contradictory and seem to vary between countries because of the differences in diet and other habits of the population and the different environmental conditions. In Greece, no analytical epidemiological study has been conducted in the past and the available descriptive data on Campylobacter infection are limited as surveillance of laboratory-confirmed cases is voluntary and only a few hospitals report on a regular basis.
The objective of the present study was to investigate risk factors for sporadic Campylobacter infections in children aged <15 years in Attica, Greece.
METHODS
Study design
This study was designed as a prospective, matched, case-control study and was conducted from December 2004 to December 2006, in Attica, an area with a population of 538 620 children aged <15 years according to the 2001 census (32% of Greece's children population).
As microbiological data show that the prevalence of different Campylobacter spp. and subtypes varies between different potential sources of infection, including different animal species, food and water [Reference Moore12, Reference Kayser13], and given the fact that the majority of clinical infections are caused by Campylobacter jejuni [Reference Lawson14], only C. jejuni infections were included in the study.
Cases
Although there is a significant private healthcare sector in Greece, accessing it was not suitable for the purposes of this study as most children with diarrhoea who visit a private paediatrician do not submit stool samples. As we needed laboratory-confirmed cases, we could not use data from the private sector. Moreover, private paediatricians refer more severe cases of gastrointestinal disease to paediatric hospitals in order to have a stool culture. Therefore, we decided to study only cases that visited the three paediatric hospitals of the study area.
The microbiological laboratories of the hospitals reported any bacteriologically verified case of C. jejuni infection to the research team. The laboratories of the three hospitals routinely culture the stool specimens submitted by the paediatricians of the hospitals for Campylobacter. Samples are taken at the emergency room or the paediatric wards from children with acute gastrointestinal symptoms (diarrhoea and fever) for the investigation of presumptive infectious enteritis according to the hospitals' protocol. Hospitalized children with other conditions, chronic or congenital diseases who may have been investigated for gastrointestinal pathogens were not included in the study. If stool cultures from more than one member of a household yielded a C. jejuni strain, only the first identified case was enrolled.
The mother or guardian of each child was questioned about potential exposures in the week prior to the onset of illness. If the mothers/guardians were unable to specify an illness onset date (11·2%), they were questioned about the 1-week period before the stool sample was submitted.
In order to reduce recall bias, cases were contacted as quickly as possible after notification from the hospitals. In case the time between the completion of the interview and the onset of the disease was more than 30 days, cases were not enrolled in the study. Children that had been abroad in the 1-week period prior to disease onset were not included in the study because of their potentially unique exposures.
Controls
Controls were selected with the cooperation of the orthopaedic departments of the three paediatric hospitals. For every case included in the study a control was picked from the registry of the orthopaedic clinic of the same hospital. In this way controls came from the same population that gave rise to the cases. Controls were children with acute orthopaedic problems (e.g. strains or minor injuries from falls). Some of them were hospitalized for a short period of time and some of them were sent home. All of them had already been discharged at the time of the interview. Controls were matched with cases by gender and age group (<1, 1–4, 5–9, 10–14 years). Children with severe orthopaedic problems, chronic or congenital diseases and surgical patients were excluded. Controls were also excluded from the study (a) if they had a history of a Campylobacter infection, (b) if it was likely that they had had a gastrointestinal infection in the preceding month (indicated by diarrhoea or abdominal pain with fever) or (c) if they had travelled abroad in the week prior to interview. If a child was not eligible for enrolment or the mother/guardian declined to participate in the study, additional controls were identified using the same method and were contacted until one control had been enrolled for each case. Controls were asked for exposures during the week immediately before the completion of the questionnaire and each control was interviewed within 1 week of the corresponding case interview.
Data collection
The study protocol and the structured questionnaire used were approved by the scientific committees of the three paediatric hospitals. Verbal informed consent was obtained from the mother/guardian of each child prior to the interview. The questionnaire was tested in a 2-month pilot study and was modified accordingly, reflecting Greek eating habits and activities. Data were collected through telephone interviews and it should be noted that all interviews were conducted by the same person, the principal researcher, who has extensive experience with data collection methods and questionnaire administration. Cases and controls were blended before the telephone interview and the interviewer was blinded to case/control status at the time of the interview. The first part of the questionnaire was exactly the same for cases and controls and the second part included all questions that were different. The questionnaire captured demographic and clinical data of the children, as well as travel history (foreign and domestic), food history (>30 exposures), eating out history (type of restaurant or takeaway), history of breast- or bottle-feeding (for infants), water consumption (tap water, bottled water, and other sources), recreational water activities, animal contacts (pets and farm animals), household contacts, outdoor leisure activities (visits to parks or farms, playing in the garden, swimming, sports), other illness and medication (antibiotics, antacids) during the 7 days before onset of illness. The food history covered meat and fish, poultry and eggs, vegetables (raw vegetables, and prepared/pre-washed salads), fruit, milk and dairy products, as well as the manner in which foods – including chicken – were purchased (fresh, frozen or ready prepared), prepared or consumed (home-cooked, takeaway, pre-cooked). The questionnaire also included questions on employment status of the parents and ethnicity as well as other socio-economic indicators (marital status, income, number of children in the household, place of residence). The criteria used for the classification of ethnicity were (a) country of birth and (b) parental country of birth for the identification of second-generation ethnic groups. In case one of the parents was Greek, ethnicity was considered as Greek. For each variable, participants were first queried about whether they had been exposed (yes/no responses). If the answer was positive, exposure frequency was recorded (e.g. number of meals consumed within the 7-days period, number of glasses drunk per day).
Data entry and statistical analysis
Data from the completed questionnaires were entered into a database and were analysed using SPSS v. 16.0 (SPSS Inc., USA). Each binomial exposure was analysed individually (univariate analysis) and Mantel–Haenszel matched odds ratios and the corresponding 95% confidence intervals were calculated. For continuous variables with Kolmogorov–Smirnov P value <0·05, Mann–Whitney U test was performed. Variables which reached a significance level of ⩽0·10 in the univariate analysis were then entered simultaneously in the conditional multivariate logistic regression (variable selection method: enter). An association was considered statistically significant when P⩽0·05 and all reported P values were two-tailed. The population-attributable risk (PAR) for the factors found to be statistically significantly associated with the risk of Campylobacter infection and the corresponding 95% confidence intervals were calculated with the use of Stata SE8 (StataCorp., USA).
RESULTS
Response rate was high for both cases and controls (97·5% and 93·6%, respectively). Nine cases were excluded from the study because they had been abroad in the 1-week period prior to disease onset (all of them had travelled to Albania). In total, 205 cases of indigenous Campylobacter infection and 205 matched controls were interviewed and were included in the analysis. The median interval between illness onset (or sample date) and completion of the questionnaire was 12 days [interquartile range (IQR) 7 days] and the median time between interviewing cases and their matched controls was 6 days (IQR 3 days).
Demographic characteristics and ethnicity
Demographic characteristics of cases and controls are shown in Table 1. According to the table there was a greater proportion of males (61·5%) in cases, a finding consistent in all age groups. The median age of cases and controls was 1 year (IQR 3 years) and 2 years (IQR 4 years), respectively. All children were born in Greece and ethnicity in the table refers to parental country of birth.
Table 1. Demographic characteristics of cases and controls (n=205)
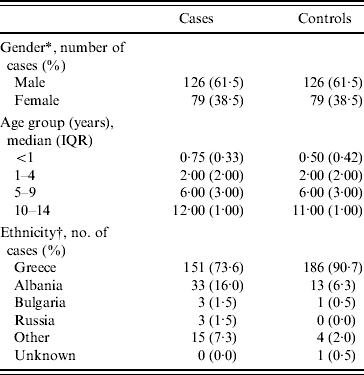
IQR, Interquartile range.
* Equal numbers as cases and controls are matched by gender.
† Parental country of birth.
Symptoms and severity of illness
Clinical symptoms and severity of infections are presented in Table 2. The 205 cases amassed in total 1265 days of illness and the 167 cases that were hospitalized accumulated 733 hospital days. Ninety-five mothers/guardians reported absence from their work for a total of 452 days (median 4 days, IQR 4 days) and 31 reported that their children were absent from school for a total of 214 days (median 5 days, IQR 7 days).
Table 2. Reported clinical symptoms and severity of illness (n=205)
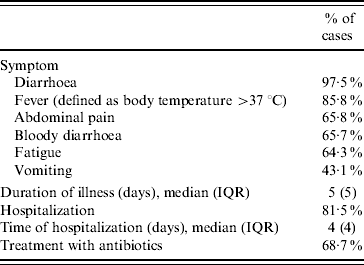
IQR, Interquartile range.
Univariate risk estimates
Ten factors with a P value <0·10 in the univariate analysis and some selected factors that have been associated with C. jejuni infection in previous studies are presented in Table 3.
Table 3. Mantel–Haenszel matched odds ratios, 95% confidence intervals, and P values for probable risk and ‘protective’ factors of Campylobacter infection
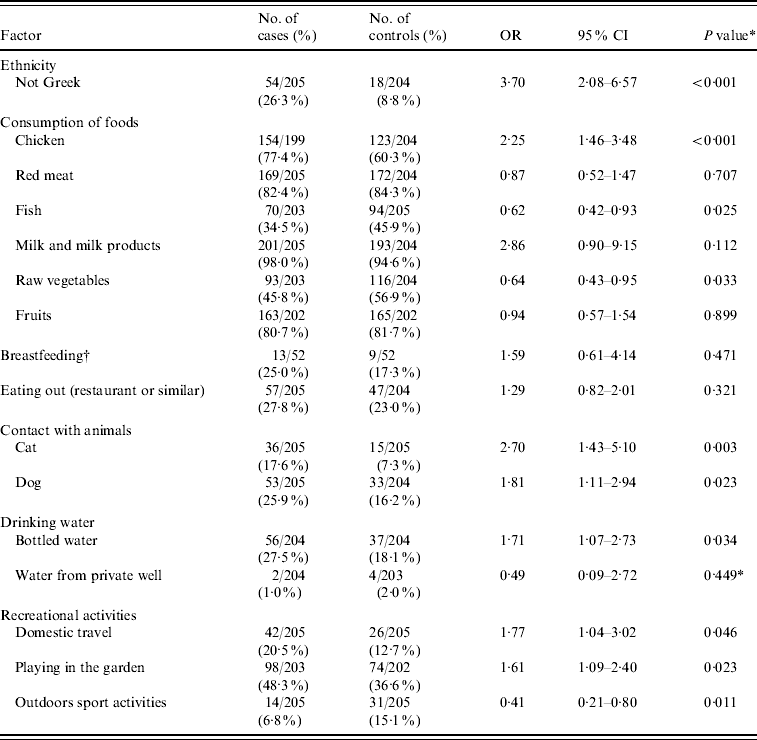
OR, odds ratio; CI, confidence interval.
* χ2 test.
† Only for children aged <1 year.
Multivariate analysis
The adjusted odds ratios with significant associations with Campylobacter infection included (a) ethnicity (Greek vs. non-Greek), (b) consumption of chicken the week prior to disease onset, (c) playing in the garden, and (d) consumption of raw vegetables (‘protective’ factor) (Table 4). Further analyses with separate variables for each type of chicken (fresh, frozen, ready prepared) and each form of consumption (home-cooked, takeaway, pre-cooked) were not significant. The evaluation on whether the association between chicken consumption and Campylobacter infection was modified by age, sex, and ethnicity showed that none of these interactions was significant. The PARs of the factors found to be statistically significantly associated with the risk of disease are presented in Table 5.
Table 4. Statistically significant results of conditional multivariate logistic regression analysis, adjusted odds ratios, 95% confidence intervals and P values
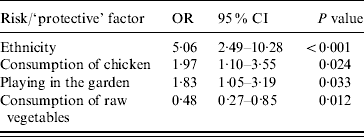
OR, Odds ratio; CI, confidence interval.
Table 5. Population attributable risks and 95% confidence intervals of exposures associated with Campylobacter infection
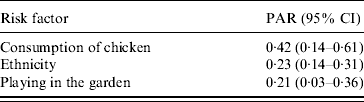
PAR, Population attributable risk; CI, confidence interval.
DISCUSSION
Our study indicates the importance of a well-recognized risk factor for Campylobacter infection; i.e. consumption of chicken the week prior to disease onset. Consumption of chicken has been identified as a risk factor for human Campylobacter infection in many of the analytical epidemiological studies conducted [Reference Fajó-Pascual6, Reference Danis15–Reference Eberhart-Phillips20].
The second most important identified risk factor was ethnicity. Belonging to an ethnic group other than Greek was a risk factor for disease according to our study results. Ethnic inequalities in Campylobacter infection have also been found in other studies but data are contradictory as to whether ethnic origin is a risk factor or just hides other behavioural and eating factors [Reference Simonsen, Frisch and Ethelberg21–23].
A possible explanation of the observed correlation of disease occurrence with playing in the garden may be the more frequent exposure of children to animal faeces. Animal contact, has been identified as a risk factor in many studies in the past [Reference Fullerton8, Reference Carrique-Mas9, Reference Tenkate and Stafford11, Reference Neimann18, Reference Kapperud24–26], but was not related to disease frequency in the multivariate analysis. Outdoor leisure activities in public parks or other private facilities designed for children were also not related to disease occurrence.
The PAR for consumption of chicken was highest, followed by ethnicity and playing in the garden. PARs are a useful tool when designing preventive programmes and setting their priorities. For example, according to the results of our study a programme aimed at educating people of different origins, regarding food-handling hygiene practices – especially during chicken preparation – could probably contribute to a reduction of Campylobacter infection incidence in children in Attica.
The finding that consumption of raw vegetables the week prior to disease onset is a ‘protective’ factor of the disease should be treated with caution because of the possibility of confounding: children who eat vegetables may generally have a healthier lifestyle, or even better economic status. However, we cannot exclude the possibility that this association is causal. Possible causal mechanisms for protection against Campylobacter infection include the effect of diet on the intestinal flora, and a boost to general immunity [Reference Kimura27]. Data regarding the consumption of vegetables are contradictory as there are also studies that have identified it as a risk factor for the disease [Reference Neimann18, Reference Evans, Ribeiro and Salmon28].
The main limitations of the current study are: (a) cases included in the study may represent a more severe spectrum of the disease and may have been exposed to a higher dose of microorganisms than mild sporadic cases in the community, (b) the focus of the study on the urban area of Attica may limit the relevance of the findings to the rest of Greece, in particular rural areas, and (c) the size of the study may not have been sufficiently large to show small differences between exposures in cases and controls. These limitations do not influence the internal validity of our study. However, the first two influence the generalization of our results. We actually believe that studying cases that visited the hospital, instead of mild sporadic cases in the community, was preferable because the contrast between these cases and the general healthy population was greater and thus it was easier to identify risk factors in a relatively small sample. In addition, risk factors for infection may be different between urban and rural areas but the majority of the Greek population live in urban areas.
Finally, another potential limitation of the study is recall bias because mothers/guardians of cases tend to better remember some of the exposures, compared to controls, as a result of their children's illness. This may have led to an overestimation of some associations. However, we do not believe this to be a serious drawback to our study because controls were also selected from the hospital population and mothers/guardians of controls were asked about exposures during the week immediately prior to the completion of the questionnaire.
The results of the current study reveal the need for further research on the effect of the identified risk factors as well as of the factors that might be hidden behind them.
DECLARATION OF INTEREST
None.







