INTRODUCTION
Human salmonellosis is one of the most common foodborne illnesses worldwide. In Japan, more than 10 000 cases of foodborne salmonellosis were reported annually from 1996 to 1999 [1]. During this period, Salmonella enterica subsp. enterica serovar Enteritidis (S. Enteritidis) was isolated most frequently in salmonellosis cases, accounting for 58% of cases in 1996, 55% in 1997, 62% in 1998, and 46% in 1999. Because food poisoning caused by S. Enteritidis was closely linked to egg consumption [1], the Enforcement Regulations of the Food Sanitation Law (Law No. 23 of 1948) were amended for safe distribution of raw shell eggs and liquid egg products in 1998. The number of foodborne salmonellosis cases decreased significantly between 2000 and 2004 [Reference Toyofuku2]. However, Salmonella remains one of the top two causative agents of bacterial food poisoning. S. Enteritidis remains the most predominant serovar, observed in more than 30% of foodborne salmonellosis cases [3].
Eggs and eggshells can be contaminated with Salmonella at the farm level. Salmonella in eggs results from infection of the oviduct. The presence of Salmonella on the shells is attributable to environmental contamination secondary to bacterial shedding from infected birds. The reduction in Salmonella prevalence in layer chickens is, therefore, one of the most effective ways to decrease Salmonella contamination of eggs. The Ministry of Agriculture, Forestry and Fisheries of Japan (MAFF) issued the ‘Guidelines on Control Measures on Salmonella in Manufacturing Feed’ [4] and ‘Guidelines on Integrated Control Measures on Salmonella in Eggs’ [5] in 1998 and 2005, respectively. Concurrently, vaccines against S. Enteritidis only and those against both S. Enteritidis and S. Typhimurium have been commercially available since 1998 and 2004, respectively. The total number of human salmonellosis incidents was 757 in 1998, and rapidly decreased to 67 in 2009, according to the statistics of food poisoning by the Ministry of Health, Labour and Welfare [6]. Although these figures imply that the control measures introduced in recent years have effectively reduced the prevalence of Salmonella in laying-hen farms, corroborative evidence is lacking, because Salmonella prevalence in laying-hen farms had never been investigated before the present study was conducted.
The objective of this study was to determine the baseline Salmonella prevalence in laying-hen farms in Japan and to examine the relationship between Salmonella presence and farm management factors. In addition, serovars and antimicrobial resistance profiles in Salmonella isolates were determined for comparison with those from human salmonellosis.
METHODS
Sampling
We asked owners of laying-hen farms to participate in the present study through the Japan Poultry Association (JPA). Overall, owners of 338 laying-hen farms voluntarily participated in this survey. Each farm kept ⩾1000 laying birds. The farms tested were located in 45 prefectures in Japan. Samples were collected from 400 flocks as follows. Five fresh, pooled caecal excrement samples and two dust samples were obtained from each house. Each fresh, pooled caecal excrement sample (~10 g) consisted of caecal excrement from several birds. Each dust sample (~25 g) was produced by mixing the primary samples obtained from the vicinity of several ventilators. The samples were collected from a laying-hen flock at the end of the laying period (within a maximum of 2 months before depopulation) (237 flocks) and/or at the beginning of egg production after induced moulting (77 flocks). Alternatively, if none of the flocks in a farm reached these production stages during the study period, samples were collected from the oldest laying-hen flock in the farm (86 flocks). Such a flexible arrangement allowed collection of as many samples as possible from the selected farms during the study period. A flock was defined as a group of birds raised in a house during the same period of time.
Each sample was placed in sterilized plastic vials and transported to the Research Institute for Animal Science in Biochemistry and Toxicology by express delivery under refrigeration. At the laboratory, the samples were kept refrigerated until examination, which was performed within a week after their arrival.
Bacteriological examination
Ten grams of the caecal sample or 25 g of the dust sample were mixed in 10 or 100 ml buffered peptone water (BPW) (Oxoid Ltd, UK), respectively. Next, 10 ml of the suspension was incubated for 24 h at 35°C for pre-enrichment in 40 ml BPW. After incubation, 0·1 and 1 ml of the culture was added to 10 ml Rappaport–Vassiliadis broth (Oxoid) and 15 ml Hajna tetrathionate broth (Eiken Kagaku, Japan), respectively, and incubated for 18–20 h at 42°C. After incubation, each culture was streaked onto two selective isolation agar plates: ES Salmonella agar II (Eiken Kagaku) and desoxycholate hydrogen sulphide lactose agar (Eiken Kagaku) containing 20 μg/ml of novobiocin (Wako Junyaku, Japan). The remaining Hajna tetrathionate broth was cultured for 5–7 days at room temperature as delayed secondary enrichment culture and then streaked onto the two selective isolation agar plates. Candidate colonies were biochemically identified. Salmonella isolates were tested by slide agglutination with O antisera (Denka Seiken Co., Japan) and tube agglutination with H antisera (Denka Seiken). Serovars were determined on the basis of reaction with O- and H-group antigens according to the Kauffmann–White scheme [Reference Poppoff7]. A flock was considered positive if Salmonella was isolated from one or more of the seven samples (five caecal samples, two dust samples) obtained.
Antimicrobial susceptibility
The minimum inhibitory concentration of various antimicrobials was determined using the agar dilution method of the Clinical and Laboratory Standards Institute (CLSI, formerly NCCLS) [8]. Enterococcus faecalis ATCC29212, Escherichia coli ATCC25922, and Staphylococcus aureus ATCC29213 were used as quality control strains. The following antimicrobials were tested: ampicillin (ABPC), cefazolin (CEZ), ceftiofur (CTF), dihydrostreptomycin (DSM), gentamicin (GM), kanamycin (KM), apramycin (APM), oxytetracycline (OTC), bicozamycin (BCM), chloramphenicol (CP), colistin (CL), nalidixic acid (NA), and enrofloxacin (ERFX). The resistant breakpoints were adopted from those defined by CLSI [9]. The breakpoints not defined by CLSI were obtained from a previous report [Reference Esaki10].
Statistical analysis
A questionnaire was conducted to examine the relationship between Salmonella prevalence and farm management factors. The risk factor analysis was performed at the farm level. If a farm had both Salmonella-positive and Salmonella-negative flocks, the farm was considered positive. A windowless house (WLH) was defined as a layer house with negative or positive pressure ventilators. During negative ventilation, fans inside the house created a negative pressure that allowed a supply of fresh air into the house from the wall. During positive pressure ventilation, fresh air was introduced by the fans. An open house (OH) had open windows for ventilation. Water supplied to the tested farms was obtained from public drinking-water systems or groundwater, either chlorinated or without any disinfection process. Public drinking water and chlorinated groundwater were defined as disinfected water for the purpose of identifying risk factors in this study.
Initially, univariate analyses were performed. If the expected value of a variable was >5, the χ2 test was used; otherwise, Fisher's exact test was used. Variables with a P value <0·25 in these tests were used for multivariate logistic regression analysis. However, if a strong association (P<0·05) in any combination of these variables was observed, one of the two variables of interest was selected to avoid multi-collinearity. A logistic regression model was then constructed using a stepwise approach with a backward elimination procedure until all the remaining variables were statistically significant (P<0·05). For this final model, two-way interactions between the remaining factors were investigated. Finally, the overall fit of the model was assessed using the Hosmer–Lemeshow goodness-of-fit test. All data analyses were performed using SPSS 17.0J (SPSS Japan Inc., Japan).
RESULTS
Salmonella prevalence and serovars
Sampling was conducted between September 2007 and March 2008. In 81·4% (275/338) of farms, samples were collected during 2 months between October and November 2007. Salmonella was isolated from 78 [19·5%, 95% confidence interval (CI) 15·6–23·4) of 400 flocks (Table 1). In the case of 41 flocks, both caecal and dust samples were Salmonella positive, whereas in the case of 14 and 23 flocks, only caecal and only dust samples, respectively, were Salmonella positive. The prevalence (16·0%, 64/400) of Salmonella based on dust samples trended higher than that (13·5%, 54/400) based on caecal samples, although the difference was not statistically significant (P=0·32). The prevalence of Salmonella was significantly (P<0·01) higher in flocks reared in WLHs than in those reared in OHs, regardless of the production stages of the sampled flocks.
Table 1. Number of Salmonella-positive flocks in 400 layer flocks
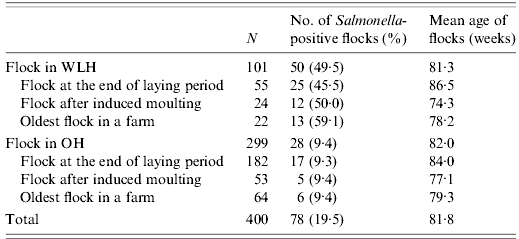
WLH, Windowless house; OH, open house.
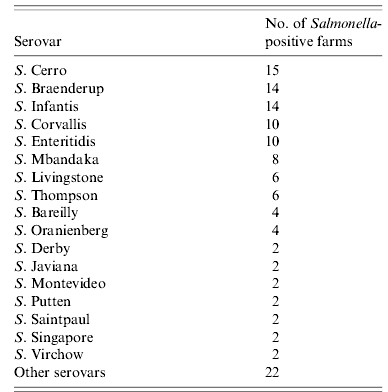
In all, 132 isolates were obtained from 70 (20·7%, 95% CI 16·4–25·0) of 338 laying-hen farms. They were serotyped into 32 serovars and five untypable Salmonella. In 35 farms, only one serovar was isolated, whereas in the remaining farms, multiple serovars were isolated (2 serovars in 21 farms, 3 serovars in 7 farms, and >3 serovars in 7 farms). Of 62 farms at which samples were obtained from a flock at the end of its laying period as well as from a flock at the beginning of egg production after induced moulting, both types of samples tested positive in eight farms. In five of these eight farms, isolated serovars (two or three per farm) were identical between the two flocks in each farm. Seventeen serovars were isolated from multiple farms (Table 2). S. Cerro was isolated from 15 farms and was the most prevalent serovar. S. Enteritidis was isolated from 10 (3·0%) farms (six WLH farms and four OH farms). S. Typhimurium was not isolated in this study.
Table 2. Frequency distribution of isolated Salmonella serovars in laying-hen farms
Antimicrobial susceptibility
Of 132 isolates, 106 (80·3%) were sensitive to all the antimicrobials tested. Resistance to 8/13 antimicrobials tested was observed (Table 3). Only five (3·8%) isolates were multi-antimicrobial resistant. Eleven S. Enteritidis isolates were obtained from 10 farms, eight (72·7%) of which were sensitive to all the antimicrobials tested and three of which were resistant to one (BCM or NA) or two (DSM and CL) antimicrobials. Fourteen S. Infantis isolates were obtained from 14 farms, 13 (92·9%) of which were sensitive to all the antimicrobials tested and one of which was resistant to OTC.
Table 3. Antimicroial resistance profiles of Salmonella isolates
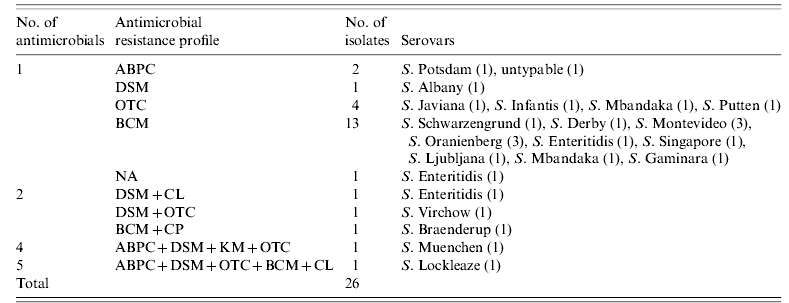
ABPC, Ampicillin; DSM, dihydrostreptomycin; OTC, oxytetracycline; BCM, bicozamycin; NA, nalidixic acid; CL, colistin; CP, chloramphenicol; KM, kanamycin.
Identification of risk factors for Salmonella presence in laying-hen farms
Of 338 farms tested in the present study, 85 (25·1%) had WLHs and constituted more than half (59·4%) of all laying hens reared in all the farms tested in this study. The average number of raised birds in a farm was 116 000 (1000–2 346 000). Of the 400 flocks, the age of 370 (92·5%) flocks was >51 weeks. The average number of chicken houses in a farm was seven (range 1–48). The mean age of the flocks sampled was 81·8 weeks (range 25·7–185·7 weeks). Complete answers to the questionnaire were returned by the owners of 313 farms. The prevalence of Salmonella in WLH farms (44/85, 51·8%) was significantly (P<0·01) higher than that in OH farms (26/253, 10·3%) [odds ratio (OR) 9·4, 95% CI 5·2–16·9]. The data derived from the questionnaire indicated that rearing and biosecurity management practices differed significantly between WLH farms and OH farms (Tables 4 and 5), with more stringent rearing and biosecurity management practices followed at the former farms. These were considered to be two distinct subpopulations with potentially different risk factors. Therefore, a separate analysis was performed for each subpopulation.
Table 4. Results of univariate analysis of risks for Salmonella prevalence in WLH farms
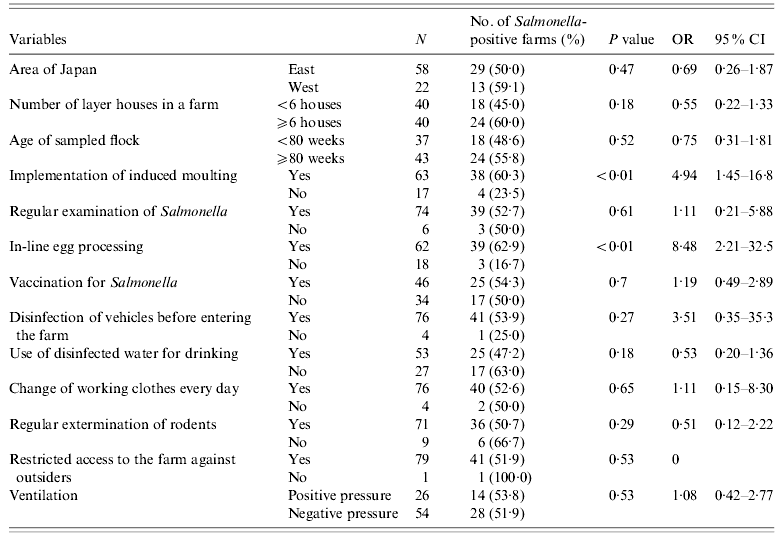
WLH, Windowless house; OR, odds ratio; CI, confidence interval.
Table 5. Results of univariate analysis of risks for Salmonella prevalence in OH farms
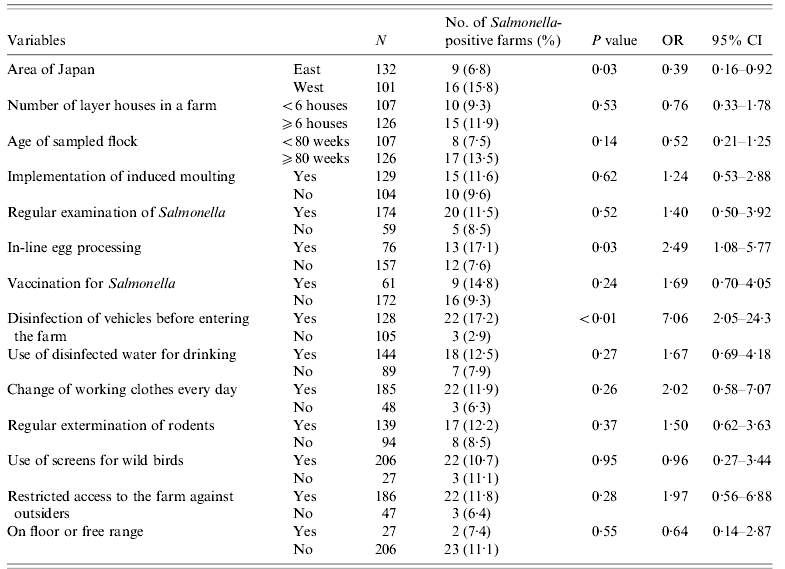
OH, Open house; OR, odds ratio; CI, confidence interval.
For the WLH farm subpopulation, 13 variables were used for univariate analysis (Table 4). Four variables (‘number of layer houses in a farm’, ‘implementation of induced moulting’, ‘in-line egg processing’, and ‘use of disinfected water for drinking’) were associated (P<0·25) with Salmonella prevalence. After the stepwise procedure, two variables, ‘implementation of induced moulting’ and ‘in-line egg processing’, remained in the final multiple logistic regression model (Table 6). The OR of Salmonella presence was significantly higher when WLH farms implemented induced moulting (OR 5·24, 95% CI 1·43–19·21, P=0·012) or in-line egg processing (OR 8·89, 95% CI 2·23–35·38, P=0·002).
Table 6. Results of a multivariate analysis of variables in associated with Salmonella prevalence in WLH farms
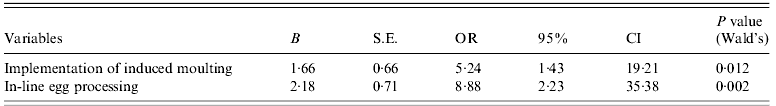
WLH, Windowless house; S.E., standard error; OR, odds ratio; CI, confidence interval.
Hosmer and Lemeshow χ2=0·313, P=0·855.
For the OH farm subpopulation, 14 variables were used for univariate analysis (Table 5). Five variables were associated (P<0·25) with Salmonella prevalence. Among the five variables, the variable ‘in-line egg processing’ was significantly (P<0·05) related to the two variables ‘area of Japan’ and ‘vaccination for Salmonella’; the variable ‘age of sampled birds’ was significantly (P<0·05) related to the variable ‘disinfection of vehicle before entering a farm’. Three variables, ‘area of Japan’, ‘vaccination for Salmonella’, and ‘disinfection of vehicle before entering a farm’, were excluded from the multivariate logistic regression analysis, because ‘in-line egg processing’ was associated with Salmonella presence in WLH farms and ‘disinfection of vehicle before entering a farm’ is a general biosecurity measure that could not promote Salmonella prevalence. After the stepwise procedure, only one variable, ‘in-line egg processing’, remained in the final multiple logistic regression model. The OR of Salmonella presence was significantly higher when OH farms implemented in-line egg processing (OR 2·49, 95% CI 1·08–5·77, P=0·03) than when they implemented off-line egg processing.
DISCUSSION
To determine the Salmonella status of laying-hen flocks, both caecal and dust samples were used in the present study. Although this study suggested the prevalence of Salmonella based on dust samples, both caecal and dust samples will be needed to determine Salmonella status, because Salmonella might be isolated only from caecal samples, as was the case with 14/78 positive flocks in this study. Iwabuchi et al. [Reference Iwabuchi11] recently reported that Salmonella was isolated from 48 (23·6%) of 203 laying-hen farms in Japan between December 2004 and March 2005. Their study used only airborne dust for Salmonella isolation. The corresponding value in our study was 16·9% (57/338 farms). A 6·7% decrease in Salmonella prevalence in laying-hen farms has been observed; this deceasing trend may reflect adherence by farmers to MAFF guidelines [4, 5] and the use of Salmonella vaccines, although the decrease is insignificant (P=0·057).
We recently isolated S. Enteritidis, S. Derby, S. Livingstone, and S. Cerro from eggshells of commercial raw shell eggs between August 2007 and January 2008 [Reference Sasaki12], around the same time as this study. These four serovars were also isolated in multiple laying-hen farms, suggesting that Salmonella contamination of shell eggs occurs at laying-hen farms or egg grading and packaging centres. The present study showed that various Salmonella serovars are sporadically found in laying-hen farms around Japan. S. Enteritidis, the fourth most common serovar, was found in 3·0% of laying-hen farms, whereas S. Typhimurium was not isolated. This profile is completely different from that in the European Union, where S. Enteritidis is by far the most common serovar, isolated in half of Salmonella-positive flocks, and S. Typhimurium was the third most common serovar in laying-hen flocks [13, 14]. Although there are no official statistics on the vaccination rate of Salmonella in laying-hen farms, vaccination was conducted in one-third (107/313) of the farms in this study. The low prevalence rates of S. Enteritidis and S. Typhimurium in laying-hen farms in Japan might indicate the presence of a vaccination-related effect in eliminating these serovars from laying-hen farms.
This study indicates that S. Infantis is the third most dominant serovar in laying-hen farms in Japan. Although S. Infantis has been reported to be an important cause of human salmonellosis in Japan [3], it is unlikely that the S. Infantis present in laying-hen farms is responsible for these cases. Most S. Infantis isolates from laying-hen farms were only resistant to OTC in the present study. S. Infantis isolates from broilers in Japan [Reference Asai15] and human isolates (T. Asai, personal communication) were frequently resistant to OTC, DSM, KM, and trimethoprim. As previously reported by Noda et al. [Reference Noda16], pulsed-field gel electrophoresis and amplified fragment length polymorphism revealed that genotypes of S. Infantis isolates from humans were similar to those from chicken meat, whereas there were no common profiles between humans and chicken egg isolates. This strongly suggests that human S. Infantis infection in Japan is caused by the consumption of broiler meat and not by the consumption of eggs.
In Japan, rates of multi-antimicrobial resistance were lower in Salmonella isolates from layer chickens than in those from cattle, pigs, and broilers [Reference Asai17]. The present study conducted between September 2007 and March 2008 showed that the resistance rate of strains to two or more of the antimicrobials was low (3·8%), and this is consistent with the findings of other studies in Japan. Asai et al. [Reference Asai17] reported that the resistance rate of Salmonella isolates from layer chickens to two or more antimicrobials was 10·7% between 2000 and 2003. We recently reported that none of Salmonella isolates from eggshells of commercial raw shell eggs were resistant to more than two antimicrobials [Reference Iwabuchi11]. These data suggest that the multi-antimicrobial resistance rate of Salmonella in laying-hen farms remains low.
The ages of 370 (92·5%) flocks tested in this study were >51 weeks. The difference in the timing of sampling in the production cycle of laying hens did not influence the prevalence of Salmonella. This may have been the result of flock-to-flock spread of Salmonella, causing indiscriminate infection, regardless of flock age. The isolation of identical sets of serovars from two flocks in a farm, observed in the case of five farms, strongly suggests the horizontal transfer of Salmonella between different flocks. In addition, considering the normal rearing period of laying hens (<2 years), the age of 51 weeks would be sufficiently advanced to detect the presence of Salmonella in laying-hen farms.
Half of WLH farms were found to be Salmonella positive. The prevalence of S. Enteritidis was statistically (P=0·02) higher in WLH farms (7·1%) than in OH farms (1·6%). Laying hens in WLH farms constituted more than half (59·4%) of all the laying hens reared in all the farms tested in this study. These results suggest that measures against Salmonella in WLH farms should be prioritized to decrease Salmonella contamination of eggs.
The present study identified ‘induced moulting’ as a risk factor associated with Salmonella presence in WLH farms. Induced moulting is generally used to stimulate multiple laying cycles and restore egg quality. Although induced moulting is an important economic tool for the egg industry, it places undue stress on birds, suppressing the immune system and creating an environment suitable for S. Enteritidis growth. Holt [Reference Holt18] reported that moulted hens excreted greater numbers of S. Enteritidis in faeces in a simulated situation. Murase et al. [Reference Murase19] showed that the levels of environmental Salmonella increased markedly in commercial flocks after induced moulting. Although discontinuation of the practice of induced moulting might effectively reduce the prevalence of Salmonella in WLH farms, it may not be a feasible risk management option, because induced moulting is widely practised in large-scale layer terms [e.g. 78·8% (63/80) of WLH farms in this study] for economic reasons. Establishment of control measures to protect layer hens from Salmonella infection during induced moulting in WLH farms is necessary.
In-line egg processing was identified as a risk factor associated with Salmonella presence in both WLH and OH farms, and this suggests that egg conveyor belts are vehicles for Salmonella, facilitating house-to-house spread once Salmonella is introduced to some houses in a farm. In Japan, all-in/all-out management at the farm level is not common; thus, it is common to find flocks of different ages in a farm. Therefore, Salmonella maintained in older flocks might invade new flocks immediately after their introduction through egg conveyors.
The results of the questionnaire survey show that WLH farms tended to apply stricter biosecurity measures than OH farms. For example, almost all of WLH farms in this study (>88%) applied biosecurity measures such as disinfection of vehicles before entering the farm, change of working clothes every day, regular extermination of rodents, and restricted access of outsiders to the farm. It is necessary to evaluate the effectiveness of these biosecurity measures in protecting laying-hen farms from the introduction of Salmonella, and to confirm that laying-hen farms rigorously implement these biosecurity measures. A preliminary examination of the effectiveness of rodent control programmes and vaccination against S. Enteritidis is provided below.
The present study did not indicate any positive impacts of the regular extermination of rodents in preventing Salmonella contamination in WLHs. Matsumoto et al. [Reference Matsumoto, Miyama and Murakami20] reported that 22 (17·9%) of 123 roof rats captured in WLHs were Salmonella positive, suggesting that roof rats are one of the most important factors in Salmonella control in WLHs. Lapuz et al. [Reference Lapuz21] also indicated that roof rats are carriers of S. Enteritidis and S. Infantis, and may have played a major role in the spread and maintenance of these pathogens inside WLHs. Furthermore, Lapuz et al. [Reference Lapuz21] reported that roof rats infected with S. Enteritidis were still observed after implementation of a rodent control programme. These findings suggest that the rodent control programme conducted in this study could not exterminate rodents. We interviewed several farmers who regularly implement rodent control programmes in WLHs regarding the effectiveness of these programmes; they indicated that (1) it is very likely that rodents play an important role in the spread and maintenance of Salmonella in poultry houses, and (2) rodent control programmes cannot decrease the numbers of rodents, not to mention exterminate them, once rodents reside in poultry houses, and are only effective in preventing their increase.
In this study, S. Enteritidis-positive flocks were found in 4/46 WLH farms conducting vaccination against only S. Enteritidis or both S. Enteritidis and S. Typhimurium. However, this result should not be construed as the ineffectiveness of vaccination against S. Enteritidis in eliminating the bacterium. It should be noted that all of these farms have implemented regular examinations for Salmonella. It is quite likely that vaccination is conducted after Salmonella is detected in these farms, giving a false impression of the impacts of Salmonella vaccination when viewed purely in terms of the correlation between vaccination records and Salmonella isolation.
Because S. Enteritidis remains the most predominant serovar in human salmonellosis in Japan, and because eggs are suspected to be the main source of human exposure, efforts to reduce human salmonellosis in Japan should focus on the establishment of control measures against S. Enteritidis in laying-hen farms. The primary focus should be on control measures to prevent the spread of Salmonella during induced moulting and/or through conveyer belts for in-line processing in WLH and OH farms.
Although there might be selection biases owing to voluntary participation, this study design is considered optimal for estimation of the prevalence of Salmonella in laying-hen farms, because at present, participation in such surveys cannot be enforced on laying-hen farmers in Japan. This study should be regarded as the first step towards the accurate estimation of the prevalence of Salmonella in laying-hen farms. It is very important to conduct comparable studies on a regular basis to enhance farmer participation and more accurately estimate the prevalence of Salmonella.
ACKNOWLEDGEMENTS
We express our gratitude to JPA and the owners of the laying-hen farms for their cooperation. This study was funded by the Ministry of Agriculture, Forestry and Fisheries of Japan.
DECLARATION OF INTEREST
None.








